Functional Organization and Dynamic Activity in the Superior Colliculus of the Echolocating Bat, Eptesicus fuscus
- PMID: 29180610
- PMCID: PMC6705813
- DOI: 10.1523/JNEUROSCI.1775-17.2017
Functional Organization and Dynamic Activity in the Superior Colliculus of the Echolocating Bat, Eptesicus fuscus
Abstract
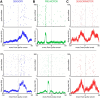
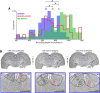
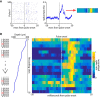
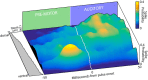
Sensory-guided behaviors require the transformation of sensory information into task-specific motor commands. Prior research on sensorimotor integration has emphasized visuomotor processes in the context of simplified orienting movements in controlled laboratory tasks rather than an animal's more complete, natural behavioral repertoire. Here, we conducted a series of neural recording experiments in the midbrain superior colliculus (SC) of echolocating bats engaged in a sonar target-tracking task that invoked dynamic active sensing behaviors. We hypothesized that SC activity in freely behaving animals would reveal dynamic shifts in neural firing patterns within and across sensory, sensorimotor, and premotor layers. We recorded neural activity in the SC of freely echolocating bats (three females and one male) and replicated the general trends reported in other species with sensory responses in the dorsal divisions and premotor activity in ventral divisions of the SC. However, within this coarse functional organization, we discovered that sensory and motor neurons are comingled within layers throughout the volume of the bat SC. In addition, as the bat increased pulse rate adaptively to increase resolution of the target location with closing distance, the activity of sensory and vocal premotor neurons changed such that auditory response times decreased, and vocal premotor lead times shortened. This finding demonstrates that SC activity can be modified dynamically in concert with adaptive behaviors and suggests that an integrated functional organization within SC laminae supports rapid and local integration of sensory and motor signals for natural, adaptive behaviors.SIGNIFICANCE STATEMENT Natural sensory-guided behaviors involve the rapid integration of information from the environment to direct flexible motor actions. The vast majority of research on sensorimotor integration has used artificial stimuli and simplified behaviors, leaving open questions about nervous system function in the context of natural tasks. Our work investigated mechanisms of dynamic sensorimotor feedback control by analyzing patterns of neural activity in the midbrain superior colliculus (SC) of an echolocating bat tracking and intercepting moving prey. Recordings revealed that sensory and motor neurons comingle within laminae of the SC to support rapid sensorimotor integration. Further, we discovered that neural activity in the bat SC changes with dynamic adaptations in the animal's echolocation behavior.
Keywords: adaptive behavior; echolocation; natural vocalizations; neuroethology; sensorimotor; superior colliculus.
Copyright © 2018 the authors 0270-6474/18/380245-12$15.00/0.
Figures


Similar articles
-
Orienting responses and vocalizations produced by microstimulation in the superior colliculus of the echolocating bat, Eptesicus fuscus.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002 Mar;188(2):89-108. doi: 10.1007/s00359-001-0275-5. Epub 2002 Feb 27. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002. PMID: 11919691
-
Dynamic representation of 3D auditory space in the midbrain of the free-flying echolocating bat.Elife. 2018 Apr 10;7:e29053. doi: 10.7554/eLife.29053. Elife. 2018. PMID: 29633711 Free PMC article.
-
Midbrain auditory selectivity to natural sounds.Proc Natl Acad Sci U S A. 2016 Mar 1;113(9):2508-13. doi: 10.1073/pnas.1517451113. Epub 2016 Feb 16. Proc Natl Acad Sci U S A. 2016. PMID: 26884152 Free PMC article.
-
Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus.Physiol Rev. 1986 Jan;66(1):118-71. doi: 10.1152/physrev.1986.66.1.118. Physiol Rev. 1986. PMID: 3511480 Review.
-
Representation of perceptual dimensions of insect prey during terminal pursuit by echolocating bats.Biol Bull. 1996 Aug;191(1):109-21. doi: 10.2307/1543071. Biol Bull. 1996. PMID: 8776847 Review.
Cited by
-
Orienting our view of the superior colliculus: specializations and general functions.Curr Opin Neurobiol. 2021 Dec;71:119-126. doi: 10.1016/j.conb.2021.10.005. Epub 2021 Nov 23. Curr Opin Neurobiol. 2021. PMID: 34826675 Free PMC article. Review.
-
A three-dimensional digital neurological atlas of the mustached bat (Pteronotus parnellii).Neuroimage. 2018 Dec;183:300-313. doi: 10.1016/j.neuroimage.2018.08.013. Epub 2018 Aug 10. Neuroimage. 2018. PMID: 30102998 Free PMC article.
-
Cyto- and myeloarchitectural brain atlas of the pale spear-nosed bat (Phyllostomus discolor) in CT Aided Stereotaxic Coordinates.Brain Struct Funct. 2020 Nov;225(8):2509-2520. doi: 10.1007/s00429-020-02138-y. Epub 2020 Sep 16. Brain Struct Funct. 2020. PMID: 32936343 Free PMC article.
-
Sensory error drives fine motor adjustment.Proc Natl Acad Sci U S A. 2022 Jul 5;119(27):e2201275119. doi: 10.1073/pnas.2201275119. Epub 2022 Jun 27. Proc Natl Acad Sci U S A. 2022. PMID: 35759672 Free PMC article.
-
Sensorimotor transformation elicits systematic patterns of activity along the dorsoventral extent of the superior colliculus in the macaque monkey.Commun Biol. 2019 Aug 2;2:287. doi: 10.1038/s42003-019-0527-y. eCollection 2019. Commun Biol. 2019. PMID: 31396567 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
