Alpha-synuclein facilitates to form short unconventional microtubules that have a unique function in the axonal transport
- PMID: 29180624
- PMCID: PMC5703968
- DOI: 10.1038/s41598-017-15575-3
Alpha-synuclein facilitates to form short unconventional microtubules that have a unique function in the axonal transport
Erratum in
-
Publisher Correction: Alpha-synuclein facilitates to form short unconventional microtubules that have a unique function in the axonal transport.Sci Rep. 2018 May 17;8(1):8019. doi: 10.1038/s41598-018-25979-4. Sci Rep. 2018. PMID: 29769584 Free PMC article.
Abstract
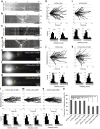
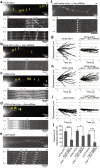
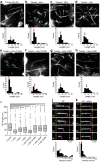
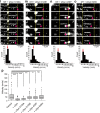
Although α-synuclein (αSyn) has been linked to Parkinson's disease (PD), the mechanisms underlying the causative role in PD remain unclear. We previously proposed a model for a transportable microtubule (tMT), in which dynein is anchored to a short tMT by LIS1 followed by the kinesin-dependent anterograde transport; however the mechanisms that produce tMTs have not been determined. Our in vitro investigations of microtubule (MT) dynamics revealed that αSyn facilitates the formation of short MTs and preferentially binds to MTs carrying 14 protofilaments (pfs). Live-cell imaging showed that αSyn co-transported with dynein and mobile βIII-tubulin fragments in the anterograde transport. Furthermore, bi-directional axonal transports are severely affected in αSyn and γSyn depleted dorsal root ganglion neurons. SR-PALM analyses further revealed the fibrous co-localization of αSyn, dynein and βIII-tubulin in axons. More importantly, 14-pfs MTs have been found in rat femoral nerve tissue, and they increased approximately 19 fold the control in quantify upon nerve ligation, indicating the unconventional MTs are mobile. Our findings indicate that αSyn facilitates to form short, mobile tMTs that play an important role in the axonal transport. This unexpected and intriguing discovery related to axonal transport provides new insight on the pathogenesis of PD.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Nitration of microtubules blocks axonal mitochondrial transport in a human pluripotent stem cell model of Parkinson's disease.FASEB J. 2018 Oct;32(10):5350-5364. doi: 10.1096/fj.201700759RR. Epub 2018 Apr 24. FASEB J. 2018. PMID: 29688812
-
Polarity sorting of axonal microtubules: a computational study.Mol Biol Cell. 2017 Nov 7;28(23):3271-3285. doi: 10.1091/mbc.E17-06-0380. Epub 2017 Oct 4. Mol Biol Cell. 2017. PMID: 28978741 Free PMC article.
-
Alpha-Synuclein affects neurite morphology, autophagy, vesicle transport and axonal degeneration in CNS neurons.Cell Death Dis. 2015 Jul 9;6(7):e1811. doi: 10.1038/cddis.2015.169. Cell Death Dis. 2015. PMID: 26158517 Free PMC article.
-
Internalization, axonal transport and release of fibrillar forms of alpha-synuclein.Neurobiol Dis. 2018 Jan;109(Pt B):219-225. doi: 10.1016/j.nbd.2017.03.007. Epub 2017 Mar 16. Neurobiol Dis. 2018. PMID: 28323023 Free PMC article. Review.
-
Microtubule acetylation dyshomeostasis in Parkinson's disease.Transl Neurodegener. 2023 May 8;12(1):20. doi: 10.1186/s40035-023-00354-0. Transl Neurodegener. 2023. PMID: 37150812 Free PMC article. Review.
Cited by
-
Disruption of axonal transport in neurodegeneration.J Clin Invest. 2023 Jun 1;133(11):e168554. doi: 10.1172/JCI168554. J Clin Invest. 2023. PMID: 37259916 Free PMC article. Review.
-
The Association between α-Synuclein and α-Tubulin in Brain Synapses.Int J Mol Sci. 2021 Aug 25;22(17):9153. doi: 10.3390/ijms22179153. Int J Mol Sci. 2021. PMID: 34502063 Free PMC article.
-
Living in Promiscuity: The Multiple Partners of Alpha-Synuclein at the Synapse in Physiology and Pathology.Int J Mol Sci. 2019 Jan 2;20(1):141. doi: 10.3390/ijms20010141. Int J Mol Sci. 2019. PMID: 30609739 Free PMC article. Review.
-
Pathological and physiological functional cross-talks of α-synuclein and tau in the central nervous system.Neural Regen Res. 2024 Apr;19(4):855-862. doi: 10.4103/1673-5374.382231. Neural Regen Res. 2024. PMID: 37843221 Free PMC article. Review.
-
α-synuclein and tau: interactions, cross-seeding, and the redefinition of synucleinopathies as complex proteinopathies.Front Neurosci. 2025 Mar 27;19:1570553. doi: 10.3389/fnins.2025.1570553. eCollection 2025. Front Neurosci. 2025. PMID: 40212715 Free PMC article. Review.
References
-
- Mitchison, T., Evans, L., Schulze, E. & Kirschner, M. Sites of microtubule assembly and disassembly in the mitotic spindle. Cell45, 515–527, doi:10.1016 (1986). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

