Crystallizable Fragment Glycoengineering for Therapeutic Antibodies Development
- PMID: 29181010
- PMCID: PMC5693878
- DOI: 10.3389/fimmu.2017.01554
Crystallizable Fragment Glycoengineering for Therapeutic Antibodies Development
Abstract
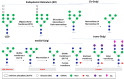
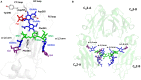
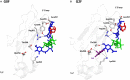
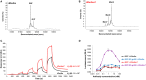
Monoclonal antibody (mAb)-based therapeutics are the fastest growing class of human pharmaceuticals. They are typically IgG1 molecules with N-glycans attached to the N297 residue on crystallizable fragment (Fc). Different Fc glycoforms impact their effector function, pharmacokinetics, stability, aggregation, safety, and immunogenicity. Fc glycoforms affect mAbs effector functions including antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) by modulating the Fc-FcγRs and Fc-C1q interactions. While the terminal galactose enhances CDC activity, the fucose significantly decreases ADCC. Defucosylated immunoglobulin Gs (IgGs) are thus highly pursued as next-generation therapeutic mAbs with potent ADCC at reduced doses. A plethora of cell glycoengineering and chemoenzymatic glycoengineering strategies is emerging to produce IgGs with homogenous glycoforms especially without core fucose. The chemoenzymatic glycosylation remodeling also offers useful avenues for site-specific conjugations of small molecule drugs onto mAbs. Herein, we review the current progress of IgG-Fc glycoengineering. We begin with the discussion of the structures of IgG N-glycans and biosynthesis followed by reviewing the impact of IgG glycoforms on antibody effector functions and the current Fc glycoengineering strategies with emphasis on Fc defucosylation. Furthermore, we briefly discuss two novel therapeutic mAbs formats: aglycosylated mAbs and Fc glycan specific antibody-drug conjugates (ADCs). The advances in the understanding of Fc glycobiology and development of novel glycoengineering technologies have facilitated the generation of therapeutic mAbs with homogenous glycoforms and improved therapeutic efficacy.
Keywords: aglycosylated monoclonal antibodies; antibody–drug conjugate; chemoenzymatic glycosylation remodeling; crystallizable fragment glycoengineering; crystallizable fragment glycosylation; effector function; homogenous glycoforms; monoclonal antibodies.
Figures



Similar articles
-
Effects of terminal galactose residues in mannose α1-6 arm of Fc-glycan on the effector functions of therapeutic monoclonal antibodies.MAbs. 2019 Jul;11(5):826-836. doi: 10.1080/19420862.2019.1608143. Epub 2019 May 8. MAbs. 2019. PMID: 30990348 Free PMC article.
-
Influence of N-glycosylation on effector functions and thermal stability of glycoengineered IgG1 monoclonal antibody with homogeneous glycoforms.MAbs. 2019 Feb/Mar;11(2):350-372. doi: 10.1080/19420862.2018.1551044. Epub 2018 Dec 10. MAbs. 2019. PMID: 30466347 Free PMC article.
-
Principles of N-Linked Glycosylation Variations of IgG-Based Therapeutics: Pharmacokinetic and Functional Considerations.Antibodies (Basel). 2020 Jun 10;9(2):22. doi: 10.3390/antib9020022. Antibodies (Basel). 2020. PMID: 32532067 Free PMC article. Review.
-
Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions.J Am Chem Soc. 2012 Jul 25;134(29):12308-18. doi: 10.1021/ja3051266. Epub 2012 Jul 16. J Am Chem Soc. 2012. PMID: 22747414 Free PMC article.
-
Importance and Monitoring of Therapeutic Immunoglobulin G Glycosylation.Exp Suppl. 2021;112:481-517. doi: 10.1007/978-3-030-76912-3_15. Exp Suppl. 2021. PMID: 34687020 Review.
Cited by
-
High-affinity CD16-polymorphism and Fc-engineered antibodies enable activity of CD16-chimeric antigen receptor-modified T cells for cancer therapy.Br J Cancer. 2019 Jan;120(1):79-87. doi: 10.1038/s41416-018-0341-1. Epub 2018 Nov 15. Br J Cancer. 2019. PMID: 30429531 Free PMC article.
-
Exploring serum and immunoglobulin G N-glycome as diagnostic biomarkers for early detection of breast cancer in Ethiopian women.BMC Cancer. 2019 Jun 17;19(1):588. doi: 10.1186/s12885-019-5817-8. BMC Cancer. 2019. PMID: 31208374 Free PMC article.
-
Appraisal of Systemic Treatment Strategies in Early HER2-Positive Breast Cancer-A Literature Review.Cancers (Basel). 2023 Aug 30;15(17):4336. doi: 10.3390/cancers15174336. Cancers (Basel). 2023. PMID: 37686612 Free PMC article. Review.
-
The role of N-glycosylation in B-cell biology and IgG activity. The aspects of autoimmunity and anti-inflammatory therapy.Front Immunol. 2023 Jul 27;14:1188838. doi: 10.3389/fimmu.2023.1188838. eCollection 2023. Front Immunol. 2023. PMID: 37575234 Free PMC article. Review.
-
Aggregation of protein therapeutics enhances their immunogenicity: causes and mitigation strategies.RSC Chem Biol. 2021 May 4;2(4):1004-1020. doi: 10.1039/d1cb00067e. eCollection 2021 Aug 5. RSC Chem Biol. 2021. PMID: 34458822 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

