Electron-deficient pyridinium salts/thiourea cooperative catalyzed O-glycosylation via activation of O-glycosyl trichloroacetimidate donors
- PMID: 29181119
- PMCID: PMC5687052
- DOI: 10.3762/bjoc.13.236
Electron-deficient pyridinium salts/thiourea cooperative catalyzed O-glycosylation via activation of O-glycosyl trichloroacetimidate donors
Abstract
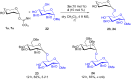
The glycosylation of O-glycosyl trichloroacetimidate donors using a synergistic catalytic system of electron-deficient pyridinium salts/aryl thiourea derivatives at room temperature is demonstrated. The acidity of the adduct formed by the 1,2-addition of alcohol to the electron-deficient pyridinium salt is increased in the presence of an aryl thiourea derivative as an hydrogen-bonding cocatalyst. This transformation occurs under mild reaction conditions with a wide range of O-glycosyl trichloroacetimidate donors and glycosyl acceptors to afford the corresponding O-glycosides in moderate to good yields with predictable selectivity. In addition, the optimized method is also utilized for the regioselective O-glycosylation by using a partially protected acceptor.
Keywords: O-glycoside; cooperative catalysis; electron-deficient pyridinium salts; regioselectivity; thiourea.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
