Diagnostic test accuracy study of 18F-sodium fluoride PET/CT, 99mTc-labelled diphosphonate SPECT/CT, and planar bone scintigraphy for diagnosis of bone metastases in newly diagnosed, high-risk prostate cancer
- PMID: 29181269
- PMCID: PMC5698615
Diagnostic test accuracy study of 18F-sodium fluoride PET/CT, 99mTc-labelled diphosphonate SPECT/CT, and planar bone scintigraphy for diagnosis of bone metastases in newly diagnosed, high-risk prostate cancer
Abstract
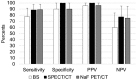
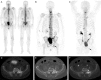
The aim of this study was to prospectively compare planar, bone scan (BS) versus SPECT/CT and NaF PET/CT in detecting bone metastases in prostate cancer. Thirty-seven consecutive, newly diagnosed, prostate cancer patients with prostate specific antigen (PSA) levels ≥ 50 ng/mL and who were considered eligible for androgen-deprivation therapy (ADT) were included in this study. BS, SPECT/CT, and NaF PET/CT, were performed prior to treatment and were repeated after six months of ADT. Baseline images from each index test were independently read by two experienced readers. The reference standard was based on a consensus decision made by a multidisciplinary team on the basis of baseline and follow-up images of the index tests, the findings of the baseline index tests by the experienced readers, and any available imaging, biochemical, and clinical data, including the response to ADT. Twenty-seven (73%) of the 37 patients had bone metastases according to the reference standard. The sensitivities for BS, SPECT/CT and NaF PET/CT were 78%, 89%, and 89%, respectively, and the specificities were 90%, 100%, and 90%, respectively. The positive predictive values of BS, SPECT/CT and NaF PET/CT were 96%, 100%, and 96%, respectively, and the negative predictive values were 60%, 77% and 75%, respectively. No statistically significant difference among the three imaging modalities was observed. All three imaging modalities showed high sensitivity and specificity. NaF PET/CT and SPECT/CT showed numerically improved, but not statistically superior, sensitivity compared with BS in this limited and selected patient cohort.
Keywords: Diagnostic test accuracy; NaF PET/CT; SPECT/CT; bone metastases; bone scan; prostate cancer.
Conflict of interest statement
None.
Figures

References
-
- Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J ESMO Guidelines Working Group. Bone health in cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2014;25(Suppl 3):iii124–137. - PubMed
-
- Graham J, Kirkbride P, Cann K, Hasler E, Prettyjohns M. Prostate cancer: summary of updated NICE guidance. BMJ. 2014;348:f7524. - PubMed
-
- Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–137. - PubMed
-
- Mohler JL, Armstrong AJ, Bahnson RR, D’Amico AV, Davis BJ, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Hurwitz M, Kane CJ, Kawachi MH, Kuettel M, Lee RJ, Meeks JJ, Penson DF, Plimack ER, Pow-Sang JM, Raben D, Richey S, Roach M 3rd, Rosenfeld S, Schaeffer E, Skolarus TA, Small EJ, Sonpavde G, Srinivas S, Strope SA, Tward J, Shead DA, Freedman-Cass DA. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. - PubMed
-
- Wondergem M, van der Zant FM, van der PT, Knol RJ. A literature review of 18F-fluoride PET/CT and 18F-choline or 11C-choline PET/CT for detection of bone metastases in patients with prostate cancer. Nucl Med Commun. 2013;34:935–945. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
