Comparative Analysis of Biopsy Upgrading in Four Prostate Cancer Active Surveillance Cohorts
- PMID: 29181514
- PMCID: PMC5752581
- DOI: 10.7326/M17-0548
Comparative Analysis of Biopsy Upgrading in Four Prostate Cancer Active Surveillance Cohorts
Abstract
Background: Active surveillance (AS) is increasingly accepted for managing low-risk prostate cancer, yet there is no consensus about implementation. This lack of consensus is due in part to uncertainty about risks for disease progression, which have not been systematically compared or integrated across AS studies with variable surveillance protocols and dropout to active treatment.
Objective: To compare risks for upgrading from a Gleason score (GS) of 6 or less to 7 or more across AS studies after accounting for differences in surveillance intervals and competing treatments and to evaluate tradeoffs of more versus less frequent biopsies.
Design: Joint statistical model of longitudinal prostate-specific antigen (PSA) levels and risks for biopsy upgrading.
Setting: Johns Hopkins University (JHU); Canary Prostate Active Surveillance Study (PASS); University of California, San Francisco (UCSF); and University of Toronto (UT) AS studies.
Patients: 2576 men aged 40 to 80 years with a GS between 2 and 6 and clinical stage T1 or T2 prostate cancer enrolled between 1995 and 2014.
Measurements: PSA levels and biopsy GSs.
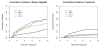
Results: After variable surveillance intervals and competing treatments were accounted for, estimated risks for biopsy upgrading were similar in the PASS and UT studies but higher in UCSF and lower in JHU studies. All cohorts had a delay of 3 to 5 months in detecting upgrading with biennial biopsies starting after a first confirmatory biopsy versus annual biopsies.
Limitation: The model does not account for possible misclassification of biopsy GS.
Conclusion: Men in different AS studies have different risks for biopsy upgrading after variable surveillance protocols and competing treatments are accounted for. Despite these differences, the consequences of more versus less frequent biopsies seem to be similar across cohorts. Biennial biopsies seem to be an acceptable alternative to annual biopsies.
Primary funding source: National Cancer Institute.
Figures

Comment in
-
Re: Comparative Analysis of Biopsy Upgrading in Four Prostate Cancer Active Surveillance Cohorts.J Urol. 2018 May;199(5):1112-1113. doi: 10.1016/j.juro.2018.02.026. Epub 2018 Feb 17. J Urol. 2018. PMID: 29677900 No abstract available.
-
Re: Comparative Analysis of Biopsy Upgrading in Four Prostate Cancer Active Surveillance Cohorts.J Urol. 2018 Sep;200(3):488-489. doi: 10.1016/j.juro.2018.05.136. Epub 2018 Jun 5. J Urol. 2018. PMID: 30412965 No abstract available.
References
-
- Chen RC, Rumble RB, Jain S. Active Surveillance for the Management of Localized Prostate Cancer (Cancer Care Ontario guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement Summary. J Oncol Pract. 2016;12(3):267–9. - PubMed
-
- Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33(3):272–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
