Determination of lesinurad in rat plasma by a UHPLC-MS/MS assay
- PMID: 29181594
- PMCID: PMC5704027
- DOI: 10.1186/s13065-017-0353-6
Determination of lesinurad in rat plasma by a UHPLC-MS/MS assay
Abstract
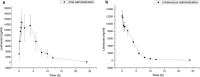
Lesinurad is an oral inhibitor of urate-anion exchanger transporter 1 and has been approved by the US Food and Drug Administration for combination therapy with a xanthine oxidase inhibitor for the treatment of hyperuricemia associated with refractory gout. In the present study, a sensitive and specific ultra high-performance liquid chromatography with tandem mass spectrometry assay was established and verified for the determination of lesinurad in rat plasma and was described in details for the first time. Chromatographic separation of lesinurad and diazepam (internal standard, IS) was performed on a Rapid Resolution HT C18 column (3.0 × 100 mm, 1.8 µm) using methanol-water (70:30, v/v) as the mobile phase at a flow rate of 0.3 mL/min. Lesinurad and IS were extracted from plasma by liquid-liquid extraction using ethyl acetate. The mass spectrometric detection was carried out using an electrospray ionization source in positive mode. Multiple reaction monitoring was used for quantification of the precursor to product ion at m/z 405.6 → 220.9 for lesinurad and m/z 285.1 → 192.8 for IS. The assay was well validated for selectivity, accuracy, precision, recovery, linearity, matrix effects, and stability. The verified method was applied to obtain the pharmacokinetic parameters and concentration-time profiles for lesinurad after oral/intravenous administration in rats. The study might provide an important reference and a necessary complement for the qualitative and quantitative evaluation of lesinurad.
Keywords: Lesinurad; Pharmacokinetics; Rat plasma; UHPLC–MS/MS.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

