Interventions for preventing oral mucositis in patients with cancer receiving treatment: cytokines and growth factors
- PMID: 29181845
- PMCID: PMC6486203
- DOI: 10.1002/14651858.CD011990.pub2
Interventions for preventing oral mucositis in patients with cancer receiving treatment: cytokines and growth factors
Abstract
Background: Oral mucositis is a side effect of chemotherapy, head and neck radiotherapy, and targeted therapy, affecting over 75% of high-risk patients. Ulceration can lead to severe pain and difficulty with eating and drinking, which may necessitate opioid analgesics, hospitalisation and supplemental nutrition. These complications may disrupt cancer therapy, which may reduce survival. There is also a risk of death from sepsis if pathogens enter the ulcers of immunocompromised patients. Ulcerative oral mucositis can be costly to healthcare systems, yet there are few preventive interventions proven to be beneficial. Cytokines and growth factors may help the regeneration of cells lining of the mouth, thus preventing or reducing oral mucositis and its negative effects.
Objectives: To assess the effects of cytokines and growth factors for preventing oral mucositis in patients with cancer who are receiving treatment.
Search methods: Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (searched 10 May 2017); the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 4) in the Cochrane Library (searched 10 May 2017); MEDLINE Ovid (1946 to 10 May 2017); Embase Ovid (7 December 2015 to 10 May 2017); CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 10 May 2017); and CANCERLIT PubMed (1950 to 10 May 2017). The US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials.
Selection criteria: We included parallel-design randomised controlled trials (RCTs) assessing the effects of cytokines and growth factors in patients with cancer receiving treatment.
Data collection and analysis: Two review authors independently screened the results of electronic searches, extracted data and assessed risk of bias. For dichotomous outcomes, we reported risk ratios (RR) and 95% confidence intervals (CI). For continuous outcomes, we reported mean differences (MD) and 95% CIs. We pooled similar studies in random-effects meta-analyses. We reported adverse effects in a narrative format.
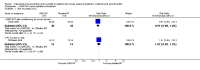
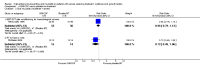
Main results: We included 35 RCTs analysing 3102 participants. Thirteen studies were at low risk of bias, 12 studies were at unclear risk of bias, and 10 studies were at high risk of bias.Our main findings were regarding keratinocyte growth factor (KGF) and are summarised as follows.There might be a reduction in the risk of moderate to severe oral mucositis in adults receiving bone marrow/stem cell transplantation after conditioning therapy for haematological cancers (RR 0.89, 95% CI 0.80 to 0.99; 6 studies; 852 participants; low-quality evidence). We would need to treat 11 adults with KGF in order to prevent one additional adult from developing this outcome (95% CI 6 to 112). There might be a reduction in the risk of severe oral mucositis in this population, but there is also some possibility of an increase in risk (RR 0.85, 95% CI 0.65 to 1.11; 6 studies; 852 participants; low-quality evidence). We would need to treat 10 adults with KGF in order to prevent one additional adult from developing this outcome (95% CI 5 to prevent the outcome to 14 to cause the outcome).There is probably a reduction in the risk of moderate to severe oral mucositis in adults receiving radiotherapy to the head and neck with cisplatin or fluorouracil (RR 0.91, 95% CI 0.83 to 1.00; 3 studies; 471 participants; moderate-quality evidence). We would need to treat 12 adults with KGF in order to prevent one additional adult from developing this outcome (95% CI 7 to infinity). It is very likely that there is a reduction in the risk of severe oral mucositis in this population (RR 0.79, 95% CI 0.69 to 0.90; 3 studies; 471 participants; high-quality evidence). We would need to treat 7 adults with KGF in order to prevent one additional adult from developing this outcome (95% CI 5 to 15).It is likely that there is a reduction in the risk of moderate to severe oral mucositis in adults receiving chemotherapy alone for mixed solid and haematological cancers (RR 0.56, 95% CI 0.45 to 0.70; 4 studies; 344 participants; moderate-quality evidence). We would need to treat 4 adults with KGF in order to prevent one additional adult from developing this outcome (95% CI 3 to 6). There might be a reduction in the risk of severe oral mucositis in this population (RR 0.30, 95% CI 0.14 to 0.65; 3 studies; 263 participants; low -quality evidence). We would need to treat 10 adults with KGF in order to prevent one additional adult from developing this outcome (95% CI 8 to 19).Due to the low volume of evidence, single-study comparisons and insufficient sample sizes, we found no compelling evidence of a benefit for any other cytokines or growth factors and there was no evidence on children. There did not appear to be any serious adverse effects of any of the interventions assessed in this review.
Authors' conclusions: We are confident that KGF is beneficial in the prevention of oral mucositis in adults who are receiving: a) radiotherapy to the head and neck with cisplatin or fluorouracil; or b) chemotherapy alone for mixed solid and haematological cancers. We are less confident about a benefit for KGF in adults receiving bone marrow/stem cell transplant after conditioning therapy for haematological cancers because of multiple factors involved in that population, such as whether or not they received total body irradiation (TBI) and whether the transplant was autologous (the patients' own cells) or allogeneic (cells from a donor). KGF appears to be a relatively safe intervention.Due to limited research, we are not confident that there are any beneficial effects of other cytokines and growth factors. There is currently insufficient evidence to draw any conclusions about the use of cytokines and growth factors in children.
Conflict of interest statement
There was no industry funding to support the undertaking of this Cochrane Review.
Philip Riley: I am a salaried member of the Cochrane Oral Health editorial team. Anne‐Marie Glenny: none known. I am Deputy Co‐ordinating Editor of Cochrane Oral Health. Helen V Worthington: none know. I am Co‐ordinating Editor of Cochrane Oral Health. Anne Littlewood: I am a salaried member of the Cochrane Oral Health editorial team. Luisa M Fernandez Mauleffinch: I am a salaried member of the Cochrane Oral Health editorial team. Jan E Clarkson: none known. I am Co‐ordinating Editor of Cochrane Oral Health. Martin G McCabe: none known. I am an Editor with Cochrane Oral Health.
Figures





















































Update of
- doi: 10.1002/14651858.CD011990
Similar articles
-
Interventions for preventing oral mucositis in patients with cancer receiving treatment: oral cryotherapy.Cochrane Database Syst Rev. 2015 Dec 23;2015(12):CD011552. doi: 10.1002/14651858.CD011552.pub2. Cochrane Database Syst Rev. 2015. PMID: 26695736 Free PMC article.
-
Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia.Cochrane Database Syst Rev. 2016 Oct 25;10(10):CD008367. doi: 10.1002/14651858.CD008367.pub3. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2020 Dec 24;12:CD008367. doi: 10.1002/14651858.CD008367.pub4. PMID: 27778318 Free PMC article. Updated.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy.Cochrane Database Syst Rev. 2017 Jul 31;7(7):CD012744. doi: 10.1002/14651858.CD012744. Cochrane Database Syst Rev. 2017. PMID: 28759701 Free PMC article.
-
Chlorhexidine mouthrinse as an adjunctive treatment for gingival health.Cochrane Database Syst Rev. 2017 Mar 31;3(3):CD008676. doi: 10.1002/14651858.CD008676.pub2. Cochrane Database Syst Rev. 2017. PMID: 28362061 Free PMC article.
Cited by
-
Cost-effectiveness randomized clinical trial on the effect of photobiomodulation therapy for prevention of radiotherapy-induced severe oral mucositis in a Brazilian cancer hospital setting.Support Care Cancer. 2021 Mar;29(3):1245-1256. doi: 10.1007/s00520-020-05607-6. Epub 2020 Jul 3. Support Care Cancer. 2021. PMID: 32621262 Clinical Trial.
-
Bovine Colostrum Treatment of Specific Cancer Types: Current Evidence and Future Opportunities.Molecules. 2022 Dec 7;27(24):8641. doi: 10.3390/molecules27248641. Molecules. 2022. PMID: 36557775 Free PMC article. Review.
-
Ice-cream used as cryotherapy during high-dose melphalan conditioning reduces oral mucositis after autologous hematopoietic stem cell transplantation.Sci Rep. 2021 Nov 18;11(1):22507. doi: 10.1038/s41598-021-02002-x. Sci Rep. 2021. PMID: 34795377 Free PMC article.
-
Expert consensus on the prevention and treatment of radiochemotherapy-induced oral mucositis.Int J Oral Sci. 2025 Jul 15;17(1):54. doi: 10.1038/s41368-025-00382-8. Int J Oral Sci. 2025. PMID: 40664676 Free PMC article.
-
Epithelial-Mesenchymal Transition Associated with Head and Neck Squamous Cell Carcinomas: A Review.Cancers (Basel). 2021 Jun 17;13(12):3027. doi: 10.3390/cancers13123027. Cancers (Basel). 2021. PMID: 34204259 Free PMC article. Review.
References
References to studies included in this review
Antoun 2009 {published data only}
-
- Antoun S, Boige V, Ducreux M, Paintain M, van't Hof M, Enslen M, et al. Protective effect of an enteral formula containing TGF‐beta(2) in the prevention of chemotherapy‐induced diarrhoea: a pilot study. e‐SPEN, European e‐Journal of Clinical Nutrition and Metabolism 2009;4(6):e348‐50.
Blazar 2006 {published data only}
Blijlevens 2013 {published data only}
-
- Blijlevens N, Château M, Krivan G, Rabitsch W, Szomor A, Pytik R, et al. In a high‐dose melphalan setting, palifermin compared to placebo had no effect on oral mucositis or related patient's burden. Supportive Care in Cancer 2011;19(Suppl 2):S227‐8. - PubMed
-
- Blijlevens N, Château M, Krivan G, Rabitsch W, Szomor A, Pytik R, et al. Medical resource use of two different dosing regimens of palifermin to prevent mucositis in multiple myeloma patients receiving one‐day administration of high‐dose melphalan. Bone Marrow Transplantation 2011;46(Suppl 1s):S163‐4.
-
- Blijlevens N, Château M, Krivan G, Rabitsch W, Szomor A, Pytik R, et al. Randomized controlled trial of two different dosing regimens of palifermin to prevent mucositis in multiple myeloma patients receiving one‐day administration of high‐dose melphalan. Blood 2010;116(21):904.
-
- Blijlevens N, Château M, Krivan G, Rabitsch W, Szomor A, Pytlik R, et al. In a high‐dose melphalan setting, palifermin compared with placebo had no effect on oral mucositis or related patient's burden. Bone Marrow Transplantation 2013;48(7):966‐71. - PubMed
Bradstock 2014 {published data only}
-
- Bradstock KF, Link E, Collins M, Iulio J, Lewis ID, Schwarer A, et al. A randomized trial of prophylactic palifermin on gastrointestinal toxicity after intensive induction therapy for acute myeloid leukaemia. British Journal of Haematology 2014;167(5):618‐25. - PubMed
Brizel 2008 {published data only}
-
- Brizel DM, Murphy BA, Rosenthal DI, Pandya KJ, Glück S, Brizel HE, et al. Phase II study of palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma. Journal of Clinical Oncology 2008;26(15):2489‐96. - PubMed
Cartee 1995 {published data only}
-
- Cartee L, Petros WP, Rosner G, Gilbert C, Moore S, Affronti M, et al. Evaluation of GM‐CSF mouthwash for prevention of chemotherapy‐induced mucositis: a randomized double‐blind dose escalating study. Blood 1993;82(10 Suppl 1):496a. [Abs No 1968] - PubMed
-
- Cartee L, Petros WP, Rosner GL, Gilbert C, Moore S, Affronti ML, et al. Evaluation of GM‐CSF mouthwash for prevention of chemotherapy‐induced mucositis: a randomized, double‐blind, dose‐ranging study. Cytokine 1995;7(5):471‐7. - PubMed
Cesaro 2013 {published data only}
-
- Cesaro S, Nesi F, Tridello G, Abate M, Panizzolo IS, Balter R, et al. A randomized, non‐inferiority study comparing efficacy and safety of a single dose of pegfilgrastim versus daily filgrastim in pediatric patients after autologous peripheral blood stem cell transplant. PLOS One 2013;8(1):e53252. - PMC - PubMed
-
- Cesaro S, Nesi F, Tridello G, Fagioli F, Abate M, Panizzolo I, et al. A non‐inferiority study comparing efficacy and safety of a single dose of pegfilgrastim versus daily filgrastim in paediatric patients after autologous peripheral blood stem cell transplant. Bone Marrow Transplantation 2012;47(Suppl 1s):S372. - PMC - PubMed
Chi 1995 {published data only}
-
- Chi KH, Chen CH, Chan WK, Chow KC, Chen SY, Yen SH, et al. Effect of granulocyte‐macrophage colony‐stimulating factor on oral mucositis in head and neck cancer patients after cisplatin, fluorouracil and leucovorin chemotherapy. Journal of Clinical Oncology 1995;13(10):2620‐8. - PubMed
-
- Chi KH, Chen CH, Chan WK, Yen SH, Liang MJ, Chou KC, et al. Effect of granulocyte‐macrophage colony‐stimulating factor (GH‐CFS) on oral mucositis in head and neck cancer patients after cisplatin, 5‐FU and leucovorin chemotherapy. Proceedings of the American Society of Clinical Oncology 1994;13:428. [Abs No 1469] - PubMed
Crawford 1999 {published data only}
-
- Crawford J, Glaspy J, Vincent M, Tomita D, Mazanet R. Effect of filgrastim (r‐metHuG‐CSF) on oral mucositis in patients with small cell lung cancer (SCLC) receiving chemotherapy (cyclophosphamide, doxorubicin and etoposide, CAE). Proceedings of the American Society of Clinical Oncology 1994;13:442. [Abs No A1523]
-
- Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, et al. Reduction by granulocyte colony‐stimulating factor of fever and neutropenia induced by chemotherapy in patients with small‐cell lung cancer. New England Journal of Medicine 1991;325(3):164‐70. - PubMed
-
- Crawford J, Tomita DK, Mazanet R, Glaspy J, Ozer H. Reduction of oral mucositis by filgrastim (r‐metHuG‐CSF) in patients receiving chemotherapy. Cytokines, Cellular and Molecular Therapy 1999;5(4):187‐93. - PubMed
Dazzi 2003 {published data only}
-
- Dazzi C, Cariello A, Giovanis P, Monti M, Vertogen B, Leoni M, et al. Prophylaxis with GM‐CSF mouthwashes does not reduce frequency and duration of severe oral mucositis in patients with solid tumors undergoing high‐dose chemotherapy with autologous peripheral blood stem cell transplantation rescue: a double‐blind, randomized, placebo‐controlled study. Annals of Oncology 2003;14(4):559‐63. - PubMed
-
- Dazzi C, Cariello A, Monti M, Giovanis P, Vertogen B, Nanni O, et al. Prophylaxis with GM‐CSF mouthwash does not reduce frequency and duration of severe oral mucositis in patients with solid tumors undergoing high dose chemotherapy with autologous PBPC rescue: a double‐blind randomized placebo‐controlled study. Annals of Oncology 2002;13 Suppl 5:167. [Abs No 617O] - PubMed
Fink 2011 {published and unpublished data}
-
- Fink G, Ihorst G, Burbeck M, Kleber M, Kohlweyer U, Engelhardt M. Prospective randomized trial of palifermin (keratinocyte growth factor) versus best supportive care measures (BSC) for the decrease of oral mucositis (OM) in lymphoma patients receiving high‐dose BEAM‐(BCNU, Etoposide, Ara‐C, Melphalan) conditioning and autologous stem‐cell transplantation (ASCT). Onkologie. Jahrestagung der Deutschen, Österreichischen und Schweizerischen Gesellschaften für Hämatologie und Onkologie; 2011 Sept‐Oct 30‐04; Basel (Switzerland). Basel (Switzerland): S Karger AG, 2011:302.
Freytes 2004 {published data only}
-
- Freytes CO, Ratanatharathorn V, Taylor C, Abboud C, Chesser N, Restrepo A, et al. Phase I/II randomized trial evaluating the safety and clinical effects of repifermin administered to reduce mucositis in patients undergoing autologous hematopoietic stem cell transplantation. Clinical Cancer Research 2004;10(24):8318‐24. - PubMed
Gholizadeh 2016 {published data only}
Henke 2011 {published data only}
-
- Henke M, Alfonsi M, Foa P, Giralt J, Bardet E, Cerezo L, et al. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo‐controlled trial. Journal of Clinical Oncology 2011;29(20):2815‐20. - PubMed
-
- Henke M, Alfonsi M, Foa P, Giralt J, Bardet E, Cerezo L, et al. Palifermin significantly reduces severe oral mucositis in subjects with resected locally advanced head and neck cancer undergoing post‐operative concurrent radio‐chemotherapy. Radiotherapy and Oncology 2008;88 Suppl 2:S152.
Hosseinjani 2017 {published data only}
-
- Hosseinjani H, Hadjibabaie M, Gholami K, Javadi M, Radfar M, Jahangard‐Rafsanjani Z, et al. The efficacy of erythropoietin mouthwash in prevention of oral mucositis in patients undergoing autologous hematopoietic SCT: a double‐blind, randomized, placebo‐controlled trial. Hematological Oncology 2017;35(1):106‐12. - PubMed
Jagasia 2012 {published data only}
-
- Jagasia MH, Abonour R, Long GD, Bolwell BJ, Laport GG, Shore TB, et al. Palifermin for the reduction of acute GVHD: a randomized, double‐blind, placebo‐controlled trial. Bone Marrow Transplantation 2012;47(10):1350‐5. - PubMed
Katano 1995 {published data only}
Kim 2017 {published data only}
-
- Kim J‐W, Kim KI, Lee HJ, Kim B‐S, Bang S‐M, Kim I, et al. Interim analysis of randomized phase II study of recombinant human epidermal growth factor on oral mucositis induced by intensive chemotherapy with stem cell transplantation for hematologic malignancies. Blood 2010;116(21):905.
-
- Kim JW, Kim MG, Lee HJ, Koh Y, Kwon JH, Kim I, et al. Topical recombinant human epidermal growth factor for oral mucositis induced by intensive chemotherapy with hematopoietic stem cell transplantation: final analysis of a randomized, double‐blind, placebo‐controlled, phase 2 trial. PLOS One 2017;12(1):e0168854. - PMC - PubMed
-
- Kim KI, Kim JW, Lee HJ, Kim BS, Bang SM, Kim I, et al. Recombinant human epidermal growth factor on oral mucositis induced by intensive chemotherapy with stem cell transplantation. American Journal of Hematology 2013;88(2):107‐12. - PubMed
Le 2011 {published data only}
-
- Le Q, Kim H, Schneider C, Muraközy G, Skladowski K, Reinisch S, et al. Palifermin reduces severe oral mucositis in subjects with locally advanced head and neck cancer undergoing chemoradiotherapy. International Journal of Radiation Oncology, Biology, Physics 2008;72 Suppl 1:S32.
-
- Le QT, Kim HE, Schneider CJ, Muraközy G, Skladowski K, Reinisch S, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo‐controlled study. Journal of Clinical Oncology 2011;29(20):2808‐14. - PubMed
Linch 1993 {published data only}
-
- Linch DC, Scarffe H, Proctor S, Chopra R, Taylor PR, Morgenstern G, et al. Randomised vehicle‐controlled dose‐finding study of glycosylated recombinant human granulocyte colony‐stimulating factor after bone marrow transplantation. Bone Marrow Transplantation 1993;11(4):307‐11. - PubMed
Lucchese 2016a {published data only}
-
- Lucchese A, Matarese G, Ghislanzoni LH, Gastaldi G, Manuelli M, Gherlone E. Efficacy and effects of palifermin for the treatment of oral mucositis in patients affected by acute lymphoblastic leukemia. Leukemia and Lymphoma 2016;57(4):820‐7. - PubMed
Lucchese 2016b {published data only}
-
- Lucchese A, Matarese G, Manuelli M, Ciuffreda C, Bassani L, Isola G, et al. Reliability and efficacy of palifermin in prevention and management of oral mucositis in patients with acute lymphoblastic leukemia: a randomized, double‐blind controlled clinical trial. Minerva Stomatologica 2016;65(1):43‐53. - PubMed
Makkonen 2000 {published data only}
-
- Makkonen TA, Minn H, Jekunen A, Vilja P, Tuominen J, Joensuu H. Granulocyte macrophage‐colony stimulating factor (GM‐CSF) and sucralfate in prevention of radiation‐induced mucositis: a prospective randomized study. International Journal of Radiation Oncology, Biology, Physics 2000;46(3):525‐34. - PubMed
-
- Minn HR, Makkonen TA, Jekunen A, Vilja P, Tuominen J, Joensuu H. Granulocyte macrophage‐colony stimulating factor (GM‐CSF) and sucralfate in prevention of radiation‐induced mucositis: a prospective randomized study. International Journal of Radiation Oncology, Biology, Physics 1999;45 Suppl 3:239. - PubMed
McAleese 2006 {published data only}
-
- McAleese JJ, Bishop KM, A'Hern R, Henk JM. Randomized phase II study of GM‐CSF to reduce mucositis caused by accelerated radiotherapy of laryngeal cancer. British Journal of Radiology 2006;79(943):608‐13. - PubMed
Meropol 2003 {published data only}
-
- Meropol NJ, Somer RA, Gutheil J, Pelley RJ, Modiano MR, Rowinsky EK, et al. Randomized phase I trial of recombinant human keratinocyte growth factor plus chemotherapy: potential role as mucosal protectant. Journal of Clinical Oncology 2003;21(8):1452‐8. - PubMed
Nemunaitis 1995 {published data only}
-
- Nemunaitis J, Rosenfeld CS, Ash R, Freedman MH, Deeg HJ, Appelbaum F, et al. Phase III randomized, double‐blind placebo‐controlled trial of rhGM‐CSF following allogeneic bone marrow transplantation. Bone Marrow Transplantation 1995;15(6):949‐54. - PubMed
Peterson 2009 {published data only}
-
- Barker NP, Peterson DE, Akhmadullina LI, Rodionova I, Sherman NZ, Gertner JM, et al. Prophylaxis of recurrent chemotherapy‐induced oral mucositis: a phase II multicenter, randomized, placebo‐controlled trial of recombinant human intestinal trefoil factor (rhITF). Journal of Clinical Oncology 2008;26:505. [Abs No 9514]
-
- Peterson DE, Barker NP, Akhmadullina LI, Rodionova I, Sherman NZ, Davidenko IS, et al. Phase II, randomized, double‐blind, placebo‐controlled study of recombinant human intestinal trefoil factor oral spray for prevention of oral mucositis in patients with colorectal cancer who are receiving fluorouracil‐based chemotherapy. Journal of Clinical Oncology 2009;27(26):4333‐8. - PubMed
Rosen 2006 {published data only}
-
- Clarke SJ, Abdi E, Davis ID, Schnell FM, Zalcberg JR, Gutheil J, et al. Recombinant human keratinocyte growth factor (rHuKGF) prevents chemotherapy‐induced mucositis in patients with advanced colorectal cancer: a randomized phase II trial. Proceedings of the American Society of Clinical Oncology 2001;20 (Pt 1):383a. [Abs No 1529]
-
- Rosen LS, Abdi E, Davis ID, Gutheil J, Schnell FM, Zalcberg J, et al. Palifermin reduces the incidence of oral mucositis in patients with metastatic colorectal cancer treated with fluorouracil‐based chemotherapy. Journal of Clinical Oncology 2006;24(33):5194‐200. - PubMed
Saarilahti 2002 {published data only}
-
- Saarilahti K, Kajanti M, Joensuu T, Kouri M, Joensuu H. Comparison of granulocyte‐macrophage colony‐stimulating factor and sucralfate mouthwashes in the prevention of radiation‐induced mucositis: a double‐blind prospective randomized phase III study. International Journal of Radiation Oncology, Biology, Physics 2002;54(2):479‐85. - PubMed
Schneider 1999 {published data only}
-
- Schneider SB, Nishimura RD, Zimmerman RP, Tran L, Shiplacoff J, Tormey M, et al. Filgrastim (r‐metHuG‐CSF) and its potential use in the reduction of radiation‐induced oropharyngeal mucositis: an interim look at a randomized, double‐blind, placebo‐controlled trial. Cytokines, Cellular and Molecular Therapy 1999;5(3):175‐80. - PubMed
Spielberger 2004 {published data only}
-
- Emmanouilides C, Spielberger R, Stiff P, Rong A, Isitt J, Bensinger W, et al. Palifermin treatment of mucositis in transplant patients reduces health resource use: phase III results. Journal of Supportive Oncology 2004;2(2):77‐8.
-
- Spielberger R, Emmanouilides C, Stiff P, Bensinger W, Gentile T, Weisdorf D, et al. Use of recombinant human keratinocyte growth factor (palifermin) can reduce severe oral mucositis in patients with hematologic malignancies undergoing autologous peripheral blood progenitor cell transplantation after radiation‐based conditioning. Journal of Supportive Oncology 2004;2(2):73‐4.
-
- Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. New England Journal of Medicine 2004;351(25):2590‐8. - PubMed
-
- Stiff P, Bensinger W, Emmanouilides C, Gentile T, Weisdorf D, Shea T, et al. Treatment of mucositis with palifermin improves patient function and results in a clinically meaningful reduction in mouth and throat soreness: phase III results. Journal of Supportive Oncology 2004;2(2):75‐6.
-
- Stiff PJ, Emmanouilides C, Bensinger WI, Gentile T, Blazar B, Shea TC, et al. Palifermin reduces patient‐reported mouth and throat soreness and improves patient functioning in the hematopoietic stem‐cell transplantation setting. Journal of Clinical Oncology 2006;24(33):5186‐93. - PubMed
Su 2006 {published data only}
-
- Su YB. Double‐blind, randomized trial of granulocyte‐colony stimulating factor (GCSF) versus (v) placebo during postoperative radiation (RT) for advanced resectable squamous cell head and neck cancer (SCCHN): impact on mucositis. Journal of Clinical Oncology 2004;22 Suppl:14S.
-
- Su YB, Vickers AJ, Zelefsky MJ, Kraus DH, Shaha AR, Shah JP, et al. Double‐blind, placebo‐controlled, randomized trial of granulocyte‐colony stimulating factor during postoperative radiotherapy for squamous head and neck cancer. Cancer Journal 2006;12(3):182‐8. - PubMed
Vadhan‐Raj 2010 {published data only}
-
- Vadhan‐Raj S, Trent J, Patel S, Zhou X, Johnson MM, Araujo D, et al. Single‐dose palifermin prevents severe oral mucositis during multicycle chemotherapy in patients with cancer: a randomised trial. Annals of Internal Medicine 2010;153(6):358‐67. - PubMed
-
- Vadhan‐Raj S, Trent JC, Patel SR, Araujo DM, Ludwig LA, Bailey D, et al. Randomized, double‐blind, placebo‐controlled study of palifermin for the prevention of mucositis in patients receiving doxorubicin‐based chemotherapy. Journal of Clinical Oncology 2008;26(15 Suppl):9547.
van der Lelie 2001 {published data only}
-
- Lelie H, Thomas BL, Oers RH, Ek‐Post M, Sjamsoedin SA, Dijk‐Overtoom ML, et al. Effect of locally applied GM‐CSF on oral mucositis after stem cell transplantation: a prospective placebo‐controlled double‐blind study. Annals of Hematology 2001;80(3):150‐4. - PubMed
Wu 2009 {published data only}
-
- Ahn Y, Wu H, Song S, Kim Y, Oh Y, Lee C, et al. The therapeutic effect of recombinant human epidermal growth factor (rhEGF) on mucositis in patients with head and neck cancer undergoing radiotherapy with or without chemotherapy: a double‐blind placebo‐controlled prospective phase II multi‐institutional study. Journal of Clinical Oncology 2008;26(15 Suppl):6021.
-
- Lee S, Song S, Kim Y, Oh Y, Lee C, Keum K, et al. The therapeutic effect of recombinant human epidermal growth factor (rhEGF) on mucositis in patients with head and neck cancer undergoing radiotherapy with or without chemotherapy: a double‐blind placebo‐controlled prospective phase II multi‐institutional clinical trial. International Journal of Radiation Oncology, Biology, Physics 2008;72(1):S32.
-
- Wu HG, Song SY, Kim YS, Oh YT, Lee CG, Keum KC, et al. Therapeutic effect of recombinant human epidermal growth factor (RhEGF) on mucositis in patients undergoing radiotherapy, with or without chemotherapy, for head and neck cancer: a double‐blind placebo‐controlled prospective phase 2 multi‐institutional clinical trial. Cancer 2009;115(16):3699‐708. - PubMed
References to studies excluded from this review
Antin 2002 {published data only}
-
- Antin JH, Lee SJ, Neuberg D, Alyea E, Soiffer RJ, Sonis S, et al. A phase I/II double‐blind, placebo‐controlled study of recombinant human interleukin‐11 for mucositis and acute GVHD prevention in allogeneic stem cell transplantation. Bone Marrow Transplantation 2002;29(5):373‐7. - PubMed
de Koning 2007 {published data only}
-
- Koning BA, Philipsen‐Geerling B, Hoijer M, Hahlen K, Buller HA, Pieters R. Protection against chemotherapy induced mucositis by TGF‐beta(2) in childhood cancer patients: results from a randomized cross‐over study. Pediatric Blood & Cancer 2007;48(5):532‐9. - PubMed
Foncuberta 2001 {published data only}
-
- Foncuberta MC, Cagnoni PJ, Brandts CH, Mandanas R, Fields K, Derigs HG, et al. Topical transforming growth factor‐beta3 in the prevention or alleviation of chemotherapy‐induced oral mucositis in patients with lymphomas or solid tumors. Journal of Immunotherapy 2001;24(4):384‐8. - PubMed
Gebbia 1994 {published data only}
-
- Gebbia V, Valenza R, Testa A, Cannata G, Borsellino N, Gebbia N. A prospective randomized trial of thymopentin versus granulocyte‐colony stimulating factor with or without thymopentin in the prevention of febrile episodes in cancer patients undergoing highly cytotoxic chemotherapy. Anticancer Research 1994;14(2B):731‐4. - PubMed
Gladkov 2013 {published data only}
-
- Gladkov O, Buchner A, Bias P, Mueller U, Elsaesser R. Chemotherapy‐associated treatment burden in breast cancer patients receiving lipegfilgrastim or pegfilgrastim: secondary efficacy data from a phase III study. Haematologica 2013;98(Suppl 1):426. - PubMed
Gordon 1993 {published data only}
-
- Gordon B, Spadinger A, Hodges E, Coccia P. Effect of granulocyte macrophage colony stimulating factor (GMCSF) on oral mucositis after autologous bone marrow transplantation. Proceedings of the American Society of Clinical Oncology 1993;12:432. [Abs No 1489] - PubMed
Horsley 2007 {published data only}
-
- Horsley P, Bauer JD, Mazkowiack R, Gardner R, Bashford J. Palifermin improves severe mucositis, swallowing problems, nutrition impact symptoms, and length of stay in patients undergoing hematopoietic stem cell transplantation. Supportive Care in Cancer 2007;15(1):105‐9. - PubMed
Hunter 2009 {published data only}
-
- Hunter A, Mahendra P, Wilson K, Fields P, Cook G, Peniker A, et al. A randomized, double‐blind, placebo‐controlled, multicenter trial of ATL‐104, a swallowable mouthwash, in patients with oral mucositis following peripheral blood stem cell transplantation. Journal of Supportive Oncology 2007;5(4 Suppl 2):52‐3.
-
- Hunter A, Mahendra P, Wilson K, Fields P, Cook G, Peniket A, et al. Treatment of oral mucositis after peripheral blood SCT with ATL‐104 mouthwash: results from a randomized, double‐blind, placebo‐controlled trial. Bone Marrow Transplantation 2009;43(7):563‐9. - PubMed
Ifrah 1999 {published data only}
-
- Ifrah N, Witz F, Jouet JP, Francois S, Lamy T, Linassier C, et al. Intensive short term therapy with granulocyte‐macrophage‐colony stimulating factor support, similar to therapy for acute myeloblastic leukemia, does not improve overall results for adults with acute lymphoblastic leukemia. American Cancer Society 1999;86(8):1496‐505. - PubMed
Iwase 1997 {published data only}
-
- Iwase M, Yoshiya M, Kakuta S, Nagumo M. Clinical trial of recombinant granulocyte colony‐stimulating factor for chemotherapy‐induced neutropenia patients with oral cancer. Journal of Oral and Maxillofacial Surgery 1997;55(8):836‐40. - PubMed
Jones 1996 {published data only}
-
- Jones SE, Schottstaedt MW, Duncan LA, Kirby RL, Good RH, Mennel RG, et al. Randomized double‐blind prospective trial to evaluate the effects of sargramostim versus placebo in a moderate‐dose fluorouracil, doxorubicin, and cyclophosphamide adjuvant chemotherapy program for stage II and III breast cancer. Journal of Clinical Oncology 1996;14(11):2976‐83. - PubMed
Karthaus 1998 {published data only}
-
- Karthaus M, Rosenthal C, Huebner G, Paul H, Elser C, Hertenstein B, et al. Effect of topical oral G‐CSF on oral mucositis: a randomised placebo‐controlled trial. Bone Marrow Transplantation 1998;22(8):781‐5. - PubMed
-
- Karthaus M, Rosenthal C, Paul H, Novotny J, Hóbner G, Fenchel K, et al. Effect of topical oral G‐CSF application on oral mucositis in high‐grade lymphoma patients treated with hd‐methotrexate (hd‐mtx) ‐ results of a randomized placebo‐controlled trial. Proceedings of the American Society of Clinical Oncology 1998;17:235.
Kubo 2016 {published data only}
Lee 2016 {published data only}
-
- Lee KH, Kim JY, Lee MH, Han HS, Lim JH, Park KU, et al. A randomized, multicenter, phase II/III study to determine the optimal dose and to evaluate the efficacy and safety of pegteograstim (GCPGC) on chemotherapy‐induced neutropenia compared to pegfilgrastim in breast cancer patients: KCSG PC10‐09. Supportive Care in Cancer 2016;24(4):1709‐17. - PMC - PubMed
Legros 1997 {published data only}
-
- Legros M, Fleury J, Bay JO, Choufi B, Basile M, Condat P, et al. rhGM‐CSF vs placebo following rhGM‐CSF‐mobilized PBPC transplantation: a phase III double‐blind randomized trial. Bone Marrow Transplantation 1997;19(3):209‐13. - PubMed
Limaye 2013 {published data only}
-
- Limaye SA, Haddad RI, Cilli F, Sonis ST, Colevas AD, Brennan MT, et al. Phase 1b, multicenter, single blinded, placebo‐controlled, sequential dose escalation study to assess the safety and tolerability of topically applied AG013 in subjects with locally advanced head and neck cancer receiving induction chemotherapy. Cancer 2013;119(24):4268‐76. - PubMed
-
- Murphy B, Limaye SA, Sonis ST, Cilli F, Brennan MT, Hu KS, et al. Phase 1B study assessing safety, tolerability and efficacy of AG013 in subjects with head and neck cancer receiving induction chemotherapy. Supportive Care in Cancer 2012;20(1 Suppl):S259. [Abs No 1078]
Nabholtz 2002 {published data only}
-
- Nabholtz JM, Cantin J, Chang J, Guevin R, Patel R, Tkaczuk K, et al. Phase III trial comparing granulocyte colony‐stimulating factor to leridistim in the prevention of neutropenic complications in breast cancer patients treated with docetaxel/doxorubicin/cyclophosphamide: results of the BCIRG 004 trial. Clinical Breast Cancer 2002;3(4):268‐75. - PubMed
NCT00360971 {published data only}
-
- NCT00360971. Palifermin in lessening oral mucositis in patients undergoing radiation therapy and chemotherapy for locally advanced head and neck cancer [A randomized, phase III, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of palifermin (NSC# 740548; IND # 6370) for the reduction of oral mucositis in patients with locally advanced head and neck cancer receiving radiation therapy with concurrent chemotherapy (followed by surgery for selected patients)]. clinicaltrials.gov/show/NCT00360971 (first received 3 August 2006).
NCT00626639 {published data only}
-
- NCT00626639. A study of palifermin for the reduction of oral mucositis in patients with locally advanced head and neck cancer receiving postoperative radiotherapy and concurrent chemotherapy [A phase 1/2 study to evaluate safety, pharmacokinetics, pharmacodynamics and preliminary efficacy of weekly doses of palifermin (rHuKGF) for the reduction of oral mucositis in subjects with locally advanced head and neck cancer (HNC) receiving postoperative radiotherapy with concurrent chemotherapy]. clinicaltrials.gov/show/NCT00626639 (first received 21 February 2008).
Pettengell 1992 {published data only}
-
- Pettengell R, Gurney H, Radford JA, Deakin DP, James R, Wilkinson PM, et al. Granulocyte colony‐stimulating factor to prevent dose‐limiting neutropenia in non‐Hodgkin's lymphoma: a randomized controlled trial. Blood 1992;80(6):1430‐6. - PubMed
Ryu 2007 {published data only}
-
- Hoffman KE, Pugh SL, James JL, Scarantino C, Movsas B, Valicenti RK, et al. The impact of concurrent granulocyte‐macrophage colony‐stimulating factor on quality of life in head and neck cancer patients: results of the randomized, placebo‐controlled Radiation Therapy Oncology Group 9901 trial. Quality of Life Research 2014;23(6):1841‐58. - PMC - PubMed
-
- Ryu JK, Swann S, LeVeque F, Scarantino CW, Johnson D, Chen A, et al. The impact of concurrent granulocyte macrophage‐colony stimulating factor on radiation‐induced mucositis in head and neck cancer patients: a double‐blind placebo‐controlled prospective phase III study by Radiation Therapy Oncology Group 9901. International Journal of Radiation Oncology, Biology, Physics 2007;67(3):643‐50. - PubMed
Throuvalas 1995 {published data only}
-
- Throuvalas N, Antonadou D, Pulizzi M, Sarris G. Evaluation of the efficacy and safety of GM‐CSF in the prophylaxis of mucositis in patients with head and neck cancer treated with RT. European Journal of Cancer 1995;31 Suppl 5:S93. [Abs No 431]
Tsurusawa 2016 {published data only}
-
- Tsurusawa M, Watanabe T, Gosho M, Mori T, Mitsui T, Sunami S, et al. Randomized study of granulocyte colony stimulating factor for childhood B‐cell non‐Hodgkin lymphoma: a report from the Japanese pediatric leukemia/lymphoma study group B‐NHL03 study. Leukemia & Lymphoma 2016;57(7):1657‐64. - PubMed
Vitale 2014 {published data only}
-
- Vitale KM, Violago L, Cofnas P, Bishop J, Jin Z, Bhatia M, et al. Impact of palifermin on incidence of oral mucositis and healthcare utilization in children undergoing autologous hematopoietic stem cell transplantation for malignant diseases. Pediatric Transplantation 2014;18(2):211‐6. - PubMed
References to studies awaiting assessment
ACTRN12606000083594 {published data only}
-
- ACTRN12606000083594. Safety and efficacy trial of whey growth factor extract for oral mucositis [A multicentre, double‐blind, placebo‐controlled, safety and efficacy trial of whey growth factor extract (WGFEA) for oral mucositis in lymphoma patients undergoing carmustine, etoposide, cytosine arabinoside and melphalan (BEAM) chemotherapy and autologous stem cell transplantation]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=1142 (first received 27 February 2006).
Antonadou 1998 {published data only}
-
- Antonadou D, Athanassiou E, Synodinou M, Koliarakis N, Panoussaki K, Karageorgis P, et al. Evaluation of the efficacy of granulocyte macrophage colony stimulating factor (GM‐CSF) in the prevention of radiation induced mucositis. Radiotherapy and Oncology 1998;48 Suppl 1:S39.
Elsaid 2001 {published data only}
-
- Elsaid A, Farouk M. Significance of anemia and role of erythropoietin in radiation induced mucositis in head and neck cancer patients. International Journal of Radiation Oncology, Biology, Physics 2001;51(3 Suppl 1):368.
Grzegorczyk 2006 {published data only}
-
- Grzegorczyk‐Jazwinska A, Dwilewicz‐Trojaczek J, Kozak I, Karakulska‐Prystupiuk E, Gorska R. Effect of locally applied G‐CSF on oral mucositis after autogeneic and allogeneic stem cell transplantation. Acta Haematologica Polonica 2006;37(2):225‐40.
-
- Grzegorczyk‐Jazwinska A, Dwilewicz‐Trojaczek J, Kozak I, Karakulska‐Prystupiuk E, Gorska R. Effect of locally applied G‐CSF on oral mucositis after autologic stem cell transplantation. Dental and Medical Problems 2004;41(4):695‐701.
Koga 2015 {published data only}
-
- Koga Y, Tsurusawa M, Watanabe T, Gosho M, Mori T, Mitsui T, et al. Randomized study of granulocyte colony stimulating factor for childhood B‐cell non‐Hodgkin lymphoma: a report from the Japanese pediatric leukemia/lymphoma study Group B‐NHL03 study. British Journal of Haematology 2015;171(Suppl S1):63‐4. - PubMed
NCT00293462 {published data only}
-
- NCT00293462. GM‐CSF mouthwash for preventing and treating mucositis in patients who are undergoing radiation therapy for head and neck cancer [Management of mucositis with GM‐CSF (sargramostim) mouthwash study protocol]. clinicaltrials.gov/show/NCT00293462 (first received 16 February 2006).
NCT00393822 {published data only}
-
- NCT00393822. A study of palifermin for the reduction of oral mucositis in subjects with stage 2B or 3 locally advanced, colon cancer [A randomized, double‐blind, placebo‐controlled study evaluating the efficacy and safety of palifermin (recombinant human keratinocyte growth factor) for reduction of oral mucositis in subjects with stage 2B or 3 locally advanced, colon cancer receiving 5‐FU and leucovorin as adjuvant therapy]. clinicaltrials.gov/show/NCT00393822 (first received 26 October 2006).
NCT02303197 {published data only}
-
- NCT02303197. Clinical trial on the efficacy and safety of Chining decoction in the treatment of radiation stomatitis [A randomized, controlled clinical trial on the efficacy and safety of Chining decoction in the treatment of radiation stomatitis]. clinicaltrials.gov/show/NCT02303197 (first received 25 November 2014).
NCT02313792 {published data only}
-
- NCT02313792. Palifermin for patients receiving hematopoietic stem cell transplantation [Efficacy, safety and quality of life of palifermin on reducing oral mucositis in patients with hematopoietic stem cell transplantation, prospective double‐blind randomized phase III trial]. clinicaltrials.gov/show/NCT02313792 (first received 5 December 2014).
Patte 2002 {published data only}
-
- Patte C, Laplanche A, Bertozzi AI, Baruchel A, Frappaz D, Schmitt C, et al. Granulocyte colony‐stimulating factor in induction treatment of children with non‐Hodgkin's lymphoma: a randomized study of the French Society of Pediatric Oncology. Journal of Clinical Oncology 2002;20(2):441‐8. - PubMed
Schuster 2007a {published data only}
-
- Schuster MW, Anaissie E, Hurd D, Bensinger W, Mason J, McCarty J, et al. Final analysis of the phase II, randomized, double‐blind, placebo‐controlled trial of single‐dose velafermin (CG53135‐05) for the prevention of oral mucositis. Journal of Supportive Oncology 2007;5(4 Suppl 2):58‐9.
Schuster 2007b {published data only}
-
- Schuster MW, Mehta J, Waller EK, Rifkin RM, Micallef I, Hurd D, et al. A randomized placebo controlled phase II trial of prevention of severe oral mucositis using single dose velafermin in patients receiving myeloablative therapy and autologous hematopoietic stem cell transplant (AHSCT). Blood 2007;110(11):615.
Shea 2007 {published data only}
-
- Shea TC, Kewalramani T, Mun Y, Jayne G, Dreiling LK. Evaluation of single‐dose palifermin to reduce oral mucositis (OM) in fractionated total body irradiation (fTBI) and high dose (HD) chemotherapy with autologous peripheral blood progenitor cell (PBPC) transplantation. Blood 2006;108(11 Part 1):875‐6.
-
- Shea TC, Kewalramani T, Mun Y, Jayne G, Dreiling LK. Evaluation of single‐dose palifermin to reduce oral mucositis in fractionated total‐body irradiation and high‐dose chemotherapy with autologous peripheral blood progenitor cell transplantation. Journal of Supportive Oncology 2007;5(4 Suppl 2):60‐2.
Spielberger 2001 {published data only}
-
- Spielberger RT, Stiff P, Emmanouilides C, Yanovich S, Bensinger W, Hedrick E, et al. Efficacy of recombinant human keratinocyte growth factor (rHuKGF) in reducing mucositis in patients with hematologic malignancies undergoing autologous peripheral blood progenitor cell transplantation (auto‐PBPCT) after radiation‐based conditioning ‐ results of a phase 2 trial. Proceedings of the American Society of Clinical Oncology 2001;20(Pt 1):7a. [Abs No 25]
Additional references
Al‐Dasooqi 2013
-
- Al‐Dasooqi N, Sonis ST, Bowen JM, Bateman E, Blijlevens N, Gibson RJ, et al. Emerging evidence on the pathobiology of mucositis. Supportive Care in Cancer 2013;21(11):3233‐41. - PubMed
Bellm 2002
-
- Bellm LA, Cunningham G, Durnell L, Eilers J, Epstein JB, Fleming T, et al. Defining clinically meaningful outcomes in the evaluation of new treatments for oral mucositis: oral mucositis patient provider advisory board. Cancer Investigation 2002;20(5‐6):793‐800. - PubMed
Boers‐Doets 2013
-
- Boers‐Doets CB, Raber‐Durlacher JE, Treister NS, Epstein JB, Arends AB, Wiersma DR, et al. Mammalian target of rapamycin inhibitor‐associated stomatitis. Future Oncology 2013;9(12):1883‐92. - PubMed
Clarke 2007
Dwan 2008
Egger 1997
Elting 2008
-
- Elting LS, Keefe DM, Sonis ST, Garden AS, Spijkervet FK, Barasch A, et al. Patient‐reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 2008;113(10):2704‐13. - PubMed
Epstein 1999
-
- Epstein JB, Emerton S, Kolbinson DA, Le ND, Phillips N, Stevenson‐Moore P, et al. Quality of life and oral function following radiotherapy for head and neck cancer. Head & Neck 1999;21(1):1‐11. - PubMed
GRADE 2004
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jensen 2014
-
- Jensen SB, Peterson DE. Oral mucosal injury caused by cancer therapies: current management and new frontiers in research. Journal of Oral Pathology and Medicine 2014;43(2):81‐90. - PubMed
Lalla 2008
Lalla 2014
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lyman 2010
-
- Lyman GH, Dale DC, Wolff DA, Culakova E, Poniewierski MS, Kuderer NM, et al. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony‐stimulating factor: a systematic review. Journal of Clinical Oncology 2010;28(17):2914‐24. - PubMed
Miller 2001
-
- Miller M, Kearney N. Oral care for patients with cancer: a review of the literature. Cancer Nursing 2001;24(4):241‐54. - PubMed
Nonzee 2008
-
- Nonzee NJ, Dandade NA, Patel U, Markossian T, Agulnik M, Argiris A, et al. Evaluating the supportive care costs of severe radiochemotherapy‐induced mucositis and pharyngitis. Cancer 2008;113(6):1446‐52. - PubMed
Peterson 2015
-
- Peterson DE, Srivastava R, Lalla RV. Oral mucosal injury in oncology patients: perspectives on maturation of a field. Oral Diseases 2015; Vol. 21, issue 2:133‐41. - PubMed
Raber‐Durlacher 2013
-
- Raber‐Durlacher JE, Bültzingslöwen I, Logan RM, Bowen J, Al‐Azri AR, Everaus H, et al. Systematic review of cytokines and growth factors for the management of oral mucositis in cancer patients. Supportive Care in Cancer 2013;21(1):343‐55. - PubMed
Riley 2017
Scully 2006
-
- Scully C, Sonis S, Diz PD. Oral mucositis. Oral Diseases 2006;12(3):229‐41. - PubMed
Sonis 2001
-
- Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem‐cell transplantation. Journal of Clinical Oncology 2001;19(8):2201‐5. - PubMed
Sonis 2004
-
- Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer‐Jensen M, et al. Perspectives on cancer therapy‐induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004;100(9 Suppl):1995‐2025. - PubMed
Sonis 2009
-
- Sonis ST. Mucositis: the impact, biology and therapeutic opportunities of oral mucositis. Oral Oncology 2009;45(12):1015‐20. - PubMed
Trotti 2003
-
- Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiotherapy and Oncology 2003;66(3):253‐62. - PubMed
Turhal 2000
-
- Turhal NS, Erdal S, Karacay S. Efficacy of treatment to relieve mucositis‐induced discomfort. Supportive Care in Cancer 2000;8(1):55‐8. - PubMed
von Bültzingslöwen 2006
-
- Bültzingslöwen I, Brennan MT, Spijkervet FK, Logan R, Stringer A, Raber‐Durlacher JE, et al. Growth factors and cytokines in the prevention and treatment of oral and gastrointestinal mucositis. Supportive Care in Cancer 2006;14(6):519‐27. - PubMed
Williamson 2005
-
- Williamson PR, Gamble C, Altman DG, Hutton JL. Outcome selection bias in meta‐analysis. Statistical Methods in Medical Research 2005;14(5):515‐24. - PubMed
Williamson 2012
Worthington 2015
-
- Worthington H, Clarkson J, Weldon J. Priority oral health research identification for clinical decision‐making. Evidence‐based Dentistry 2015;16(3):69‐71. - PubMed
References to other published versions of this review
Riley 2015
-
- Riley P, Glenny AM, Worthington HV, Littlewood A, Clarkson JE, McCabe MG. Interventions for preventing oral mucositis in patients with cancer receiving treatment: cytokines and growth factors. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD011990] - DOI - PMC - PubMed
Worthington 2011
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

