Wearable sensors: modalities, challenges, and prospects
- PMID: 29182185
- PMCID: PMC5771841
- DOI: 10.1039/c7lc00914c
Wearable sensors: modalities, challenges, and prospects
Abstract
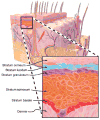
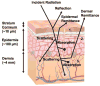
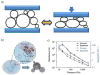
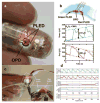
Wearable sensors have recently seen a large increase in both research and commercialization. However, success in wearable sensors has been a mix of both progress and setbacks. Most of commercial progress has been in smart adaptation of existing mechanical, electrical and optical methods of measuring the body. This adaptation has involved innovations in how to miniaturize sensing technologies, how to make them conformal and flexible, and in the development of companion software that increases the value of the measured data. However, chemical sensing modalities have experienced greater challenges in commercial adoption, especially for non-invasive chemical sensors. There have also been significant challenges in making significant fundamental improvements to existing mechanical, electrical, and optical sensing modalities, especially in improving their specificity of detection. Many of these challenges can be understood by appreciating the body's surface (skin) as more of an information barrier than as an information source. With a deeper understanding of the fundamental challenges faced for wearable sensors and of the state-of-the-art for wearable sensor technology, the roadmap becomes clearer for creating the next generation of innovations and breakthroughs.
Conflict of interest statement
The co-author (Heikenfeld) discloses a potential conflict of interest as he is a co-founder of Eccrine Systems Inc. which is commercializing sweat bio-sensing technologies. John Rogers is involved with several companies that are developing wearable technologies. Joseph Wang has no competing financial interests to disclosed. No potential conflict of interests exist for Tingrui Pan or Michelle Khine.
Figures










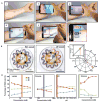
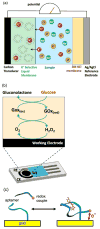
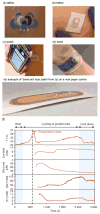
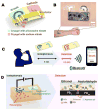

References
-
- Clarke SF, Foster JR. Br J Biomed Sci. 2012;69:83–93. - PubMed
-
- Miller J. Inventing the Apollo Spaceflight Biomedical Sensors. https://airandspace.si.edu/stories/editorial/inventing-apollo-spacefligh....
-
- Cunningham DD. In Vivo Glucose Sensing. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2010. pp. 191–215.
-
- Proksch E, Brandner JM, Jensen J-M. Exp Dermatol. 2008;17:1063–1072. - PubMed
-
- Deli MA. Biochim Biophys Acta - Biomembr. 2009;1788:892–910. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

