Graphene Oxide-Silver Nanoparticles Nanocomposite Stimulates Differentiation in Human Neuroblastoma Cancer Cells (SH-SY5Y)
- PMID: 29182571
- PMCID: PMC5751152
- DOI: 10.3390/ijms18122549
Graphene Oxide-Silver Nanoparticles Nanocomposite Stimulates Differentiation in Human Neuroblastoma Cancer Cells (SH-SY5Y)
Abstract
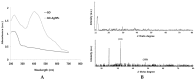
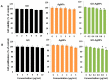
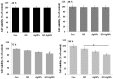
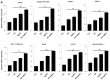
Recently, graphene and graphene related nanocomposite receive much attention due to high surface-to-volume ratio, and unique physiochemical and biological properties. The combination of metallic nanoparticles with graphene-based materials offers a promising method to fabricate novel graphene-silver hybrid nanomaterials with unique functions in biomedical nanotechnology, and nanomedicine. Therefore, this study was designed to prepare graphene oxide (GO) silver nanoparticles (AgNPs) nanocomposite (GO-AgNPs) containing two different nanomaterials in single platform with distinctive properties using luciferin as reducing agents. In addition, we investigated the effect of GO-AgNPs on differentiation in SH-SY5Y cells. The synthesized GO-AgNPs were characterized by ultraviolet-visible absorption spectroscopy (UV-vis), X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and Raman spectroscopy. The differentiation was confirmed by series of cellular and biochemical assays. The AgNPs were distributed uniformly on the surface of graphene oxide with an average size of 25 nm. As prepared GO-AgNPOs induces differentiation by increasing the expression of neuronal differentiation markers and decreasing the expression of stem cell markers. The results indicated that the redox biology involved the expression of various signaling molecules, which play an important role in differentiation. This study suggests that GO-AgNP nanocomposite could stimulate differentiation of SH-SY5Y cells. Furthermore, understanding the mechanisms of differentiation of neuroblastoma cells could provide new strategies for cancer and stem cell therapies. Therefore, these studies suggest that GO-AgNPs could target specific chemotherapy-resistant cells within a tumor.
Keywords: apoptosis; differentiation; luciferin; neuroblastoma; neuronal markers; stem cell markers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Yu A.L., Gilman A.L., Ozkaynak M.F., London W.B., Kreissman S.G., Chen H.X., Smith M., Anderson B., Villablanca J.G., Matthay K.K., et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

