Pharmacokinetics of Chlorin e₆-Cobalt Bis(Dicarbollide) Conjugate in Balb/c Mice with Engrafted Carcinoma
- PMID: 29182594
- PMCID: PMC5751159
- DOI: 10.3390/ijms18122556
Pharmacokinetics of Chlorin e₆-Cobalt Bis(Dicarbollide) Conjugate in Balb/c Mice with Engrafted Carcinoma
Abstract
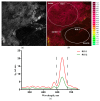
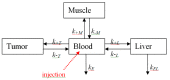
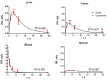
The necessary precondition for efficient boron neutron capture therapy (BNCT) is control over the content of isotope 10B in the tumor and normal tissues. In the case of boron-containing porphyrins, the fluorescent part of molecule can be used for quantitative assessment of the boron content. Study Objective: We performed a study of the biodistribution of the chlorin e₆-Cobalt bis(dicarbollide) conjugate in carcinoma-bearing Balb/c mice using ex vivo fluorescence imaging, and developed a mathematical model describing boron accumulation and release based on the obtained experimental data. Materials and Methods: The study was performed on Balb/c tumor-bearing mice (CT-26 tumor model). A solution of the chlorin e₆-Cobalt bis(dicarbollide) conjugate (CCDC) was injected into the blood at a dose of 10 mg/kg of the animal's weight. Analysis of the fluorescence signal intensity was performed at several time points by spectrofluorimetry in blood and by laser scanning microscopy in muscle, liver, and tumor tissues. The boron content in the same samples was determined by mass spectroscopy with inductively coupled plasma. Results: Analysis of a linear approximation between the fluorescence intensity and boron content in the tissues demonstrated a satisfactory value of approximation reliability with a Spearman's rank correlation coefficient of r = 0.938, p < 0.01. The dynamics of the boron concentration change in various organs, calculated on the basis of the fluorescence intensity, enabled the development of a model describing the accumulation of the studied compound and its distribution in tissues. The obtained results reveal a high level of correspondence between the model and experimental data.
Keywords: MS-ICP; boron content; boron neutron capture therapy; chlorin e6 derivatives; fluorescent microscopy; simple multichamber model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hawthorne M.F. The role of chemistry in the development of boron neutron capture therapy of cancer. Angew. Chem. Int. Ed. 1993;32:950–984. doi: 10.1002/anie.199309501. - DOI
-
- Sivaev I.B., Bregadze V.I. Polyhedral boranes for medical applications: Current status and perspectives. Eur. J. Inorg. Chem. 2009:1433–1450. doi: 10.1002/ejic.200900003. - DOI
-
- Sauerwein W.A.G., Wittig A., Moss R., Nakagawa Y., editors. Neutron Capture Therapy. Principles and Applications. Springer Science & Business Media; Berlin, Germany: 2012. p. 554.
-
- Hosmane N.S., Maguire J.A., Zhu Y., Takagi M. Boron and Gadolinium Neutron Capture Therapy for Cancer Treatment. World Scientific Publishing; Singapore: 2012. p. 246.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

