Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial
- PMID: 29183074
- PMCID: PMC5820695
- DOI: 10.1001/jama.2017.17077
Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial
Abstract
Importance: Fecal microbiota transplantation (FMT) is effective in preventing recurrent Clostridium difficile infection (RCDI). However, it is not known whether clinical efficacy differs by route of delivery.
Objective: To determine whether FMT by oral capsule is noninferior to colonoscopy delivery in efficacy.
Design, setting, and participants: Noninferiority, unblinded, randomized trial conducted in 3 academic centers in Alberta, Canada. A total of 116 adult patients with RCDI were enrolled between October 2014 and September 2016, with follow-up to December 2016. The noninferiority margin was 15%.
Interventions: Participants were randomly assigned to FMT by capsule or by colonoscopy at a 1:1 ratio.
Main outcomes and measures: The primary outcome was the proportion of patients without RCDI 12 weeks after FMT. Secondary outcomes included (1) serious and minor adverse events, (2) changes in quality of life by the 36-Item Short Form Survey on a scale of 0 (worst possible quality of life) to 100 (best quality of life), and (3) patient perception on a scale of 1 (not at all unpleasant) to 10 (extremely unpleasant) and satisfaction on a scale of 1 (best) to 10 (worst).
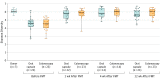
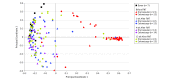
Results: Among 116 patients randomized (mean [SD] age, 58 [19] years; 79 women [68%]), 105 (91%) completed the trial, with 57 patients randomized to the capsule group and 59 to the colonoscopy group. In per-protocol analysis, prevention of RCDI after a single treatment was achieved in 96.2% in both the capsule group (51/53) and the colonoscopy group (50/52) (difference, 0%; 1-sided 95% CI, -6.1% to infinity; P < .001), meeting the criterion for noninferiority. One patient in each group died of underlying cardiopulmonary illness unrelated to FMT. Rates of minor adverse events were 5.4% for the capsule group vs 12.5% for the colonoscopy group. There was no significant between-group difference in improvement in quality of life. A significantly greater proportion of participants receiving capsules rated their experience as "not at all unpleasant" (66% vs 44%; difference, 22% [95% CI, 3%-40%]; P = .01).
Conclusions and relevance: Among adults with RCDI, FMT via oral capsules was not inferior to delivery by colonoscopy for preventing recurrent infection over 12 weeks. Treatment with oral capsules may be an effective approach to treating RCDI.
Trial registration: clinicaltrials.gov Identifier: NCT02254811.
Conflict of interest statement
Figures

Comment in
-
Capsules for Fecal Microbiota Transplantation in Recurrent Clostridium difficile Infection: The New Way Forward or a Tough Pill to Swallow?JAMA. 2017 Nov 28;318(20):1979-1980. doi: 10.1001/jama.2017.17969. JAMA. 2017. PMID: 29183052 Free PMC article. No abstract available.
-
Therapy: Oral capsule FMT effective for C. difficile infection.Nat Rev Gastroenterol Hepatol. 2018 Feb;15(2):68. doi: 10.1038/nrgastro.2017.188. Epub 2018 Jan 4. Nat Rev Gastroenterol Hepatol. 2018. PMID: 29300048 No abstract available.
-
Fecal transplant by oral capsule was noninferior to delivery by colonoscopy for C difficile recurrence.Ann Intern Med. 2018 Mar 20;168(6):JC31. doi: 10.7326/ACPJC-2018-168-6-031. Ann Intern Med. 2018. PMID: 29554669 No abstract available.
-
Microbiota replacement for Clostridium difficile by capsule is as effective as via colonoscopy.J Thorac Dis. 2018 Apr;10(Suppl 9):S1081-S1083. doi: 10.21037/jtd.2018.04.18. J Thorac Dis. 2018. PMID: 29849203 Free PMC article. No abstract available.
References
-
- Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. - PubMed
-
- Louie TJ, Miller MA, Mullane KM, et al. ; OPT-80-003 Clinical Study Group . Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431. - PubMed
-
- Wilcox MH, Gerding DN, Poxton IR, et al. ; MODIFY I and MODIFY II Investigators . Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376(4):305-317. - PubMed
-
- Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108(4):500-508. - PubMed
-
- Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48(8):693-702. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

