A proposal for the withdrawal of inhaled corticosteroids in the clinical practice of chronic obstructive pulmonary disease
- PMID: 29183382
- PMCID: PMC5706374
- DOI: 10.1186/s12931-017-0682-y
A proposal for the withdrawal of inhaled corticosteroids in the clinical practice of chronic obstructive pulmonary disease
Abstract
According to the current clinical practice guidelines for chronic obstructive pulmonary disease (COPD), the addition of inhaled corticosteroids (ICS) to long-acting β2 agonist therapy is recommended in patients with moderate-to-severe disease and an increased risk of exacerbations. However, ICS are largely overprescribed in clinical practice, and most patients are unlikely to benefit from long-term ICS therapy.Evidence from recent randomized-controlled trials supports the hypothesis that ICS can be safely and effectively discontinued in patients with stable COPD and in whom ICS therapy may not be indicated, without detrimental effects on lung function, health status, or risk of exacerbations. This article summarizes the evidence supporting the discontinuation of ICS therapy, and proposes an algorithm for the implementation of ICS withdrawal in patients with COPD in clinical practice.Given the increased risk of potentially serious adverse effects and complications with ICS therapy (including pneumonia), the use of ICS should be limited to the minority of patients in whom the treatment effects outweigh the risks.
Keywords: Algorithm; Chronic obstructive pulmonary disease; Exacerbations; Inhaled corticosteroids; Lung function.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
MM has received speaker fees from Boehringer Ingelheim, AstraZeneca, Chiesi, GlaxoSmithKline, Menarini, Teva, Grifols, and Novartis, and consulting fees from Boehringer Ingelheim, GlaxoSmithKline, Gebro Pharma, CLS Behring, Cipla, MediImmune, Mereo Biopharma, Teva, Novartis, and Grifols; BGC has received grants from SEPAR (Sociedad Española de Neumología y Cirugíatorácica), Boehringer Ingelheim, and Menarini, personal fees from AstraZeneca, Rovi, and Esteve, grants and personal fees from Novartis and Chiesi; AA has received grants from SEPAR, Esteve, and NEUMOSUR (Asociación de Neumología y Cirugía Torácica del Sur), and speaker fees from Boehringer Ingelheim, AstraZeneca, Chiesi, Gebro Pharma, Grifols, MSD, Mundipharma, Pfizer, and Novartis; MC has received personal fees from Laboratorios Menarini, GSK, and Rovi, and grants from Boehringer Ingelheim; Bernardino Alcázar-Navarrete has received personal fees from GSK, Gebro, and AstraZeneca, grants, personal fees, and non-financial support from Novartis AG and Laboratorios Menarini, and personal fees and non-financial support from Boehringer Ingelheim and Chiesi; CG has received grants from SVN (Sociedad Valenciana de Neumología) and Laboratorios Menarini; grants and speaker fees from Boehringer Ingelheim, Novartis, Rovi, and Teva, speaker fees from AstraZeneca, and grants and consulting fees from Esteve; CE has no conflicts of interest to declare; JAT has received speaker fees from Boehringer Ingelheim, Menarini, Teva, and Novartis, and consulting fees from Boehringer Ingelheim, GlaxoSmithKline, and Esteve; JMRGM has received speaker fees from Boehringer Ingelheim, AstraZeneca, Chiesi, Teva, and Novartis, and consulting fees from Boehringer Ingelheim and Teva; JAQJ has received speaker fees from Esteve, Teva, Gebro, and Grifols, and consulting fees from Boehringer Ingelheim and Teva; and Adolfo Baloira has received speaker fees from Boehringer Ingelheim, AstraZeneca, Chiesi, GlaxoSmithKline, Esteve, Ferrer, Menarini, Teva, Grifols, and Novartis, and consulting fees from Boehringer Ingelheim, GlaxoSmithKline, Esteve, Teva, Novartis, and Grifols.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
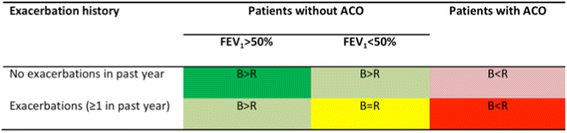
Figures

References
-
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol. 2017;53:128–149. - PubMed
-
- Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012:CD006829. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

