Depolarization and electrical stimulation enhance in vitro and in vivo sensory axon growth after spinal cord injury
- PMID: 29183676
- PMCID: PMC5752127
- DOI: 10.1016/j.expneurol.2017.11.011
Depolarization and electrical stimulation enhance in vitro and in vivo sensory axon growth after spinal cord injury
Abstract
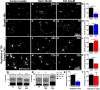
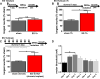
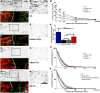
Activity dependent plasticity is a key mechanism for the central nervous system (CNS) to adapt to its environment. Whether neuronal activity also influences axonal regeneration in the injured CNS, and whether electrical stimulation (ES) can activate regenerative programs in the injured CNS remains incompletely understood. Using KCl-induced depolarization, in vivo ES followed by ex-vivo neurite growth assays and ES after spinal cord lesions and cell grafting, we aimed to identify parameters important for ES-enhanced neurite growth and axonal regeneration. Using cultures of sensory neurons, neurite growth was analyzed after KCl-induced depolarization for 1-72h. Increased neurite growth was detected after short-term stimulation and after longer stimulation if a sufficient delay between stimulation and growth measurements was provided. After in vivo ES (20Hz, 2× motor threshold, 0.2ms, 1h) of the intact sciatic nerve in adult Fischer344 rats, sensory neurons showed a 2-fold increase in in vitro neurite length one week later compared to sham animals, an effect not observed one day after ES. Longer ES (7h) and repeated ES (7days, 1h each) also increased growth by 56-67% one week later, but provided no additional benefit. In vivo growth of dorsal column sensory axons into a graft of bone marrow stromal cells 4weeks after a cervical spinal cord lesion was also enhanced with a single post-injury 1h ES of the intact sciatic nerve and was also observed after repeated ES without inducing pain-like behavior. While ES did not result in sensory functional recovery, our data indicate that ES has time-dependent influences on the regenerative capacity of sensory neurons and might further enhance axonal regeneration in combinatorial approaches after SCI.
Keywords: CNS plasticity; Electrical stimulation; axonal regeneration; dorsal root ganglion; spinal cord injury.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
none
Figures

Similar articles
-
Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice.J Neurosci. 2004 May 5;24(18):4432-43. doi: 10.1523/JNEUROSCI.2245-02.2004. J Neurosci. 2004. PMID: 15128857 Free PMC article.
-
Exercise dependent increase in axon regeneration into peripheral nerve grafts by propriospinal but not sensory neurons after spinal cord injury is associated with modulation of regeneration-associated genes.Exp Neurol. 2016 Feb;276:72-82. doi: 10.1016/j.expneurol.2015.09.004. Epub 2015 Sep 12. Exp Neurol. 2016. PMID: 26366525 Free PMC article.
-
Intraneural Injection of ATP Stimulates Regeneration of Primary Sensory Axons in the Spinal Cord.J Neurosci. 2018 Feb 7;38(6):1351-1365. doi: 10.1523/JNEUROSCI.1660-17.2017. Epub 2017 Dec 26. J Neurosci. 2018. PMID: 29279307 Free PMC article.
-
Electrical Stimulation as a Tool to Promote Plasticity of the Injured Spinal Cord.J Neurotrauma. 2020 Sep 15;37(18):1933-1953. doi: 10.1089/neu.2020.7033. Epub 2020 Jul 8. J Neurotrauma. 2020. PMID: 32438858 Free PMC article. Review.
-
Intervention strategies to enhance anatomical plasticity and recovery of function after spinal cord injury.Adv Neurol. 1997;72:257-75. Adv Neurol. 1997. PMID: 8993704 Review.
Cited by
-
Advances in Conductive Hydrogel for Spinal Cord Injury Repair and Regeneration.Int J Nanomedicine. 2023 Dec 6;18:7305-7333. doi: 10.2147/IJN.S436111. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38084124 Free PMC article. Review.
-
Enhancing rehabilitation and functional recovery after brain and spinal cord trauma with electrical neuromodulation.Curr Opin Neurol. 2019 Dec;32(6):828-835. doi: 10.1097/WCO.0000000000000750. Curr Opin Neurol. 2019. PMID: 31567546 Free PMC article. Review.
-
Alginate-Based Smart Materials and Their Application: Recent Advances and Perspectives.Top Curr Chem (Cham). 2021 Nov 23;380(1):3. doi: 10.1007/s41061-021-00360-8. Top Curr Chem (Cham). 2021. PMID: 34812965 Review.
-
MiR-20a Plays a Key Regulatory Role in the Repair of Spinal Cord Dorsal Column Lesion via PDZ-RhoGEF/RhoA/GAP43 Axis in Rat.Cell Mol Neurobiol. 2019 Jan;39(1):87-98. doi: 10.1007/s10571-018-0635-0. Epub 2018 Nov 13. Cell Mol Neurobiol. 2019. PMID: 30426336 Free PMC article.
-
Electrical stimulation for the treatment of spinal cord injuries: A review of the cellular and molecular mechanisms that drive functional improvements.Front Cell Neurosci. 2023 Feb 3;17:1095259. doi: 10.3389/fncel.2023.1095259. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36816852 Free PMC article. Review.
References
-
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in the excitability of rat dorsal root ganglion neurons. J Neurophysiol. 2001;85:630–643. - PubMed
-
- Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21:1355–1370. - PubMed
-
- Agnew WF, McCreery DB, Yuen TG, Bullara LA. Histologic and physiologic evaluation of electrically stimulated peripheral nerve: considerations for the selection of parameters. Ann Biomed Eng. 1989;17:39–60. - PubMed
-
- Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000a;12:4381–4390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

