Mesenchymal lineage cells and their importance in B lymphocyte niches
- PMID: 29183783
- PMCID: PMC11488667
- DOI: 10.1016/j.bone.2017.11.018
Mesenchymal lineage cells and their importance in B lymphocyte niches
Abstract
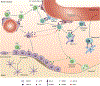
Early B lymphopoiesis occurs in the bone marrow and is reliant on interactions with numerous cell types in the bone marrow microenvironment, particularly those of the mesenchymal lineage. Each cellular niche that supports the distinct stages of B lymphopoiesis is unique. Different cell types and signaling molecules are important for the progressive stages of B lymphocyte differentiation. Cells expressing CXCL12 and IL-7 have long been recognized as having essential roles in facilitating progression through stages of B lymphopoiesis. Recently, a number of other factors that extrinsically mediate B lymphopoiesis (positively or negatively) have been identified. In addition, the use of transgenic mouse models to delete specific genes in mesenchymal lineage cells has further contributed to our understanding of how B lymphopoiesis is regulated in the bone marrow. This review will cover the current understanding of B lymphocyte niches in the bone marrow and key extrinsic molecules and signaling pathways involved in these niches, with a focus on how mesenchymal lineage cells regulate B lymphopoiesis.
Keywords: B lymphocytes; B lymphopoiesis; Mesenchymal cells; Osteoblasts; Osteoclasts.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
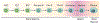

Similar articles
-
Interactions between B lymphocytes and the osteoblast lineage in bone marrow.Calcif Tissue Int. 2013 Sep;93(3):261-8. doi: 10.1007/s00223-013-9753-3. Epub 2013 Jul 10. Calcif Tissue Int. 2013. PMID: 23839529 Free PMC article. Review.
-
Retinoic Acid Receptor γ Activity in Mesenchymal Stem Cells Regulates Endochondral Bone, Angiogenesis, and B Lymphopoiesis.J Bone Miner Res. 2018 Dec;33(12):2202-2213. doi: 10.1002/jbmr.3558. Epub 2018 Aug 8. J Bone Miner Res. 2018. PMID: 30040873
-
Regulation of murine B lymphopoiesis by stromal cells.Immunol Rev. 2021 Jul;302(1):47-67. doi: 10.1111/imr.12973. Epub 2021 May 17. Immunol Rev. 2021. PMID: 34002391 Review.
-
Granulocyte colony-stimulating factor reprograms bone marrow stromal cells to actively suppress B lymphopoiesis in mice.Blood. 2015 May 14;125(20):3114-7. doi: 10.1182/blood-2015-02-629444. Epub 2015 Mar 26. Blood. 2015. PMID: 25814527 Free PMC article.
-
Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways.Proc Natl Acad Sci U S A. 2008 Nov 4;105(44):16976-81. doi: 10.1073/pnas.0802898105. Epub 2008 Oct 28. Proc Natl Acad Sci U S A. 2008. PMID: 18957542 Free PMC article.
Cited by
-
The Bone Marrow Microenvironment in B-Cell Development and Malignancy.Cancers (Basel). 2022 Apr 22;14(9):2089. doi: 10.3390/cancers14092089. Cancers (Basel). 2022. PMID: 35565219 Free PMC article. Review.
-
Treatment of B-cell precursor acute lymphoblastic leukemia with the Galectin-1 inhibitor PTX008.J Exp Clin Cancer Res. 2018 Mar 27;37(1):67. doi: 10.1186/s13046-018-0721-7. J Exp Clin Cancer Res. 2018. PMID: 29580262 Free PMC article.
-
Interactions of B-lymphocytes and bone cells in health and disease.Bone. 2023 Mar;168:116296. doi: 10.1016/j.bone.2021.116296. Epub 2021 Dec 21. Bone. 2023. PMID: 34942359 Free PMC article. Review.
-
Immunomodulation Effect of Biomaterials on Bone Formation.J Funct Biomater. 2022 Jul 25;13(3):103. doi: 10.3390/jfb13030103. J Funct Biomater. 2022. PMID: 35893471 Free PMC article. Review.
-
Regulation of Energy Metabolism during Early B Lymphocyte Development.Int J Mol Sci. 2018 Jul 27;19(8):2192. doi: 10.3390/ijms19082192. Int J Mol Sci. 2018. PMID: 30060475 Free PMC article. Review.
References
-
- Sims NA and Gooi JH, Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol, 2008. 19(5): p. 444–51. - PubMed
-
- Hardy RR, B-1 B cell development. J Immunol, 2006. 177(5): p. 2749–54. - PubMed
-
- Nagasawa T, Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol, 2006. 6(2): p. 107–16. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

