17β-estradiol and tamoxifen protect mice from manganese-induced dopaminergic neurotoxicity
- PMID: 29183790
- PMCID: PMC5905416
- DOI: 10.1016/j.neuro.2017.11.008
17β-estradiol and tamoxifen protect mice from manganese-induced dopaminergic neurotoxicity
Abstract
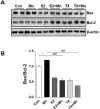
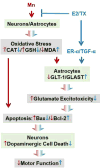
Chronic exposure to manganese (Mn) causes neurotoxicity, referred to as manganism, with common clinical features of parkinsonism. 17β-estradiol (E2) and tamoxifen (TX), a selective estrogen receptor modulator (SERM), afford neuroprotection in several neurological disorders, including Parkinson's disease (PD). In the present study, we tested if E2 and TX attenuate Mn-induced neurotoxicity in mice, assessing motor deficit and dopaminergic neurodegeneration. We implanted E2 and TX pellets in the back of the neck of ovariectomized C57BL/6 mice two weeks prior to a single injection of Mn into the striatum. One week later, we assessed locomotor activity and molecular mechanisms by immunohistochemistry, real-time quantitative PCR, western blot and enzymatic biochemical analyses. The results showed that both E2 and TX attenuated Mn-induced motor deficits and reversed the Mn-induced loss of dopaminergic neurons in the substantia nigra. At the molecular level, E2 and TX reversed the Mn-induced decrease of (1) glutamate aspartate transporter (GLAST) and glutamate transporter 1 (GLT-1) mRNA and protein levels; (2) transforming growth factor-α (TGF-α) and estrogen receptor-α (ER-α) protein levels; and (3) catalase (CAT) activity and glutathione (GSH) levels, and Mn-increased (1) malondialdehyde (MDA) levels and (2) the Bax/Bcl-2 ratio. These results indicate that E2 and TX afford protection against Mn-induced neurotoxicity by reversing Mn-reduced GLT1/GLAST as well as Mn-induced oxidative stress. Our findings may offer estrogenic agents as potential candidates for the development of therapeutics to treat Mn-induced neurotoxicity.
Keywords: 17β-estradiol; GLAST; GLT-1; Manganese; Tamoxifen; Tyrosine hydroxylase.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

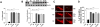
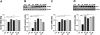
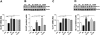
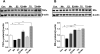
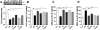
References
-
- Armagan G, Kanit L, Terek CM, Sozmen EY, Yalcin A. The levels of glutathione and nitrite-nitrate and the expression of Bcl-2 mRNA in ovariectomized rats treated by raloxifene against kainic acid. Int J Neurosci. 2009;119(2):227–239. - PubMed
-
- Avila DS, Gubert P, Fachinetto R, Wagner C, Aschner M, Rocha JB, Soares FA. Involvement of striatal lipid peroxidation and inhibition of calcium influx into brain slices in neurobehavioral alterations in a rat model of short-term oral exposure to manganese. Neurotoxicology. 2008;29(6):1062–1068. - PubMed
-
- Barbeau A. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias) Neurotoxicology. 1984;5(1):13–35. - PubMed
-
- Bourque M, Dluzen DE, Di Paolo T. Neuroprotective actions of sex steroids in Parkinson's disease. Front Neuroendocrinol. 2009;30(2):142–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

