Contact lens to measure individual ion concentrations in tears and applications to dry eye disease
- PMID: 29183834
- PMCID: PMC5817005
- DOI: 10.1016/j.ab.2017.11.014
Contact lens to measure individual ion concentrations in tears and applications to dry eye disease
Abstract
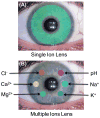
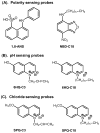
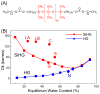
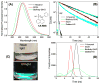
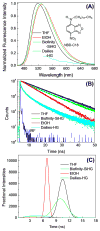
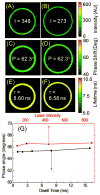
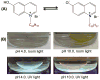
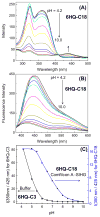
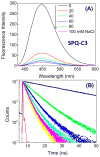
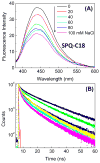
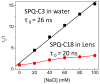
Dry eye disease (DED) affects millions of individuals in the United States and worldwide, and the incidence is increasing with an aging population. There is widespread agreement that the measurement of total tear osmolarity is the most reliable test, but this procedure provides only the total ionic strength and does not provide the concentration of each ionic species in tears. Here, we describe an approach to determine the individual ion concentrations in tears using modern silicone hydrogel (SiHG) contact lenses. We made pH (or H3O+, hydronium cation,/OH-, hydroxyl ion) and chloride ion (two of the important electrolytes in tear fluid) sensitive SiHG contact lenses. We attached hydrophobic C18 chains to water-soluble fluorescent probes for pH and chloride. The resulting hydrophobic ion sensitive fluorophores (H-ISF) bind strongly to SiHG lenses and could not be washed out with aqueous solutions. Both H-ISFs provide measurements which are independent of total intensity by use of wavelength-ratiometric measurements for pH or lifetime-based sensing for chloride. Our approach can be extended to fabricate a contact lens which provides measurements of the six dominant ionic species in tears. This capability will be valuable for research into the biochemical processes causing DED, which may improve the ability to diagnose the various types of DED.
Keywords: Chloride ion; Contact lens; Dry eye disease; Electrolytes; Fluorescence sensors; Hydrogels; Hydronium ion; Silicone hydrogels.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Fluorescent contact lens for continuous non-invasive measurements of sodium and chloride ion concentrations in tears.Anal Biochem. 2020 Nov 1;608:113902. doi: 10.1016/j.ab.2020.113902. Epub 2020 Aug 12. Anal Biochem. 2020. PMID: 32800702 Free PMC article.
-
Sodium-Sensitive Contact Lens for Diagnostics of Ocular Pathologies.Sens Actuators B Chem. 2021 Mar 15;331:129434. doi: 10.1016/j.snb.2021.129434. Epub 2021 Jan 9. Sens Actuators B Chem. 2021. PMID: 33551571 Free PMC article.
-
Glucose-sensitive silicone hydrogel contact lens toward tear glucose monitoring.J Biomed Opt. 2018 May;23(5):1-9. doi: 10.1117/1.JBO.23.5.057005. J Biomed Opt. 2018. PMID: 29774672 Free PMC article.
-
Ion channels in dry eye disease.Indian J Ophthalmol. 2023 Apr;71(4):1215-1226. doi: 10.4103/IJO.IJO_3020_22. Indian J Ophthalmol. 2023. PMID: 37026252 Free PMC article. Review.
-
Contact lens interactions with the tear film.Exp Eye Res. 2013 Dec;117:88-98. doi: 10.1016/j.exer.2013.07.013. Epub 2013 Jul 23. Exp Eye Res. 2013. PMID: 23886658 Review.
Cited by
-
Fluorescent contact lens for continuous non-invasive measurements of sodium and chloride ion concentrations in tears.Anal Biochem. 2020 Nov 1;608:113902. doi: 10.1016/j.ab.2020.113902. Epub 2020 Aug 12. Anal Biochem. 2020. PMID: 32800702 Free PMC article.
-
Wireless Non-Invasive Monitoring of Cholesterol Using a Smart Contact Lens.Adv Sci (Weinh). 2022 Oct;9(28):e2203597. doi: 10.1002/advs.202203597. Epub 2022 Aug 17. Adv Sci (Weinh). 2022. PMID: 35975449 Free PMC article.
-
Big data and machine learning for materials science.Discov Mater. 2021;1(1):12. doi: 10.1007/s43939-021-00012-0. Epub 2021 Apr 19. Discov Mater. 2021. PMID: 33899049 Free PMC article. Review.
-
Molecular and physical technologies for monitoring fluid and electrolyte imbalance: A focus on cancer population.Clin Transl Med. 2021 Jun;11(6):e461. doi: 10.1002/ctm2.461. Clin Transl Med. 2021. PMID: 34185420 Free PMC article. Review.
-
Modular Fabrication of Intelligent Material-Tissue Interfaces for Bioinspired and Biomimetic Devices.Prog Mater Sci. 2019 Dec;106:100589. doi: 10.1016/j.pmatsci.2019.100589. Epub 2019 Jul 17. Prog Mater Sci. 2019. PMID: 32189815 Free PMC article.
References
-
- Phadatare SP, Momin M, Nighojkar P, Askarkar S, Singh KK. A comprehensive review on dry eye diseases: diagnosis, medical management, recent developments, and future challenges. Adv Pharmac. 2015:1–12. article ID 704946.
-
- Schein OD, Munoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124:723–728. - PubMed
-
- Smith JA. The epidemiology of dry eye disease. Acta Ophthalmol. 2007;85(240)
-
- Smith JA., Chair Report of the Epidemiology Subcommittee, Intl. Dry Eye Workshop (2007) DEWS Epidemiology. 2007:93–107.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

