Antinociceptive effects of mixtures of mu opioid receptor agonists and cannabinoid receptor agonists in rats: Impact of drug and fixed-dose ratio
- PMID: 29183835
- PMCID: PMC5766417
- DOI: 10.1016/j.ejphar.2017.11.038
Antinociceptive effects of mixtures of mu opioid receptor agonists and cannabinoid receptor agonists in rats: Impact of drug and fixed-dose ratio
Abstract
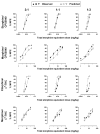
Pain is a significant clinical problem, and there is a need for effective pharmacotherapies with fewer adverse effects than currently available drugs (e.g., mu opioid receptor agonists). Cannabinoid receptor agonists enhance the antinociceptive effects of mu opioid receptor agonists, but it remains unclear which drugs and in what proportion will yield the most effective and safest treatments. The antinociceptive effects of the mu opioid receptor agonists etorphine and morphine alone and in combination with the cannabinoid receptor agonists Δ9-THC and CP55940 were studied in male Sprague-Dawley rats (n = 16) using a warm water tail withdrawal procedure. The ratio of opioid to cannabinoid (3:1, 1:1, and 1:3) varied for each mixture. Drugs administered alone or as pairwise mixtures of an opioid and a cannabinoid dose-dependently increased tail withdrawal latency. Mixtures with morphine produced supra-additive (CP55940) and additive (Δ9-THC) effects, whereas mixtures with etorphine and either cannabinoid were sub-additive. The interactions were not different among ratios for a particular mixture. The nature of the interaction between opioids and cannabinoids with regard to antinociceptive effects varies with the particular drugs in the mixture, which can have implications for designing combination therapies for pain.
Keywords: Antinociception; Cannabinoid receptor agonist; Drug-drug interactions; Rats; Thermal nociception; mu opioid receptor agonist.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures
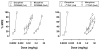
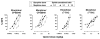
Similar articles
-
Additive antinociceptive effects of mixtures of the κ-opioid receptor agonist spiradoline and the cannabinoid receptor agonist CP55940 in rats.Behav Pharmacol. 2016 Feb;27(1):69-72. doi: 10.1097/FBP.0000000000000184. Behav Pharmacol. 2016. PMID: 26292184 Free PMC article.
-
Interactions between cannabinoid receptor agonists and mu opioid receptor agonists in rhesus monkeys discriminating fentanyl.Eur J Pharmacol. 2016 Aug 5;784:199-206. doi: 10.1016/j.ejphar.2016.05.018. Epub 2016 May 13. Eur J Pharmacol. 2016. PMID: 27184925 Free PMC article.
-
Impact of efficacy at the μ-opioid receptor on antinociceptive effects of combinations of μ-opioid receptor agonists and cannabinoid receptor agonists.J Pharmacol Exp Ther. 2014 Nov;351(2):383-9. doi: 10.1124/jpet.114.216648. Epub 2014 Sep 5. J Pharmacol Exp Ther. 2014. PMID: 25194020 Free PMC article.
-
Cannabinoid-opioid interactions during neuropathic pain and analgesia.Curr Opin Pharmacol. 2010 Feb;10(1):80-6. doi: 10.1016/j.coph.2009.09.009. Epub 2009 Oct 25. Curr Opin Pharmacol. 2010. PMID: 19857996 Free PMC article. Review.
-
Therapeutic potential of opioid/cannabinoid combinations in humans: Review of the evidence.Eur Neuropsychopharmacol. 2020 Jul;36:206-216. doi: 10.1016/j.euroneuro.2020.03.002. Epub 2020 Apr 6. Eur Neuropsychopharmacol. 2020. PMID: 32273144 Free PMC article. Review.
Cited by
-
Modulation of Morphine Analgesia, Antinociceptive Tolerance, and Mu-Opioid Receptor Binding by the Cannabinoid CB2 Receptor Agonist O-1966.Front Pharmacol. 2022 Apr 21;13:803331. doi: 10.3389/fphar.2022.803331. eCollection 2022. Front Pharmacol. 2022. PMID: 35529434 Free PMC article.
-
Comparison of null models for combination drug therapy reveals Hand model as biochemically most plausible.Sci Rep. 2019 Feb 28;9(1):3002. doi: 10.1038/s41598-019-38907-x. Sci Rep. 2019. PMID: 30816136 Free PMC article.
-
Opioid-sparing effect of cannabinoids for analgesia: an updated systematic review and meta-analysis of preclinical and clinical studies.Neuropsychopharmacology. 2022 Jun;47(7):1315-1330. doi: 10.1038/s41386-022-01322-4. Epub 2022 Apr 22. Neuropsychopharmacology. 2022. PMID: 35459926 Free PMC article.
-
Interactions between opioids and cannabinoids: Economic demand for opioid/cannabinoid mixtures.Drug Alcohol Depend. 2020 Jul 1;212:108043. doi: 10.1016/j.drugalcdep.2020.108043. Epub 2020 May 12. Drug Alcohol Depend. 2020. PMID: 32497977 Free PMC article.
-
Antinociceptive and Abuse Potential Effects of Cannabinoid/Opioid Combinations in a Chronic Pain Model in Rats.Brain Sci. 2019 Nov 17;9(11):328. doi: 10.3390/brainsci9110328. Brain Sci. 2019. PMID: 31744226 Free PMC article.
References
-
- Biscaia M, Fernández B, Higuera-Matas A, Miguéns M, Viveros MP, García-Lecumberri C, Ambrosio E. Sex-dependent effects of periadolescent exposure to the cannabinoid agonist CP-55,940 on morphine self-administration behaviour and the endogenous opioid system. Neuropharmacology. 2008;54:863–873. doi: 10.1016/j.neuropharm.2008.01.006. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

