Viability testing and transplantation of marginal livers (VITTAL) using normothermic machine perfusion: study protocol for an open-label, non-randomised, prospective, single-arm trial
- PMID: 29183928
- PMCID: PMC5719273
- DOI: 10.1136/bmjopen-2017-017733
Viability testing and transplantation of marginal livers (VITTAL) using normothermic machine perfusion: study protocol for an open-label, non-randomised, prospective, single-arm trial
Abstract
Introduction: The use of marginal or extended criteria donor livers is increasing. These organs carry a greater risk of initial dysfunction and early failure, as well as inferior long-term outcomes. As such, many are rejected due to a perceived risk of use and use varies widely between centres. Ex situ normothermic machine perfusion of the liver (NMP-L) may enable the safe transplantation of organs that meet defined objective criteria denoting their high-risk status and are currently being declined for use by all the UK transplant centres.
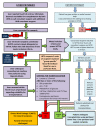
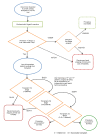
Methods and analysis: Viability testing and transplantation of marginal livers is an open-label, non-randomised, prospective, single-arm trial designed to determine whether currently unused donor livers can be salvaged and safely transplanted with equivalent outcomes in terms of patient survival. The procured rejected livers must meet predefined criteria that objectively denote their marginal condition. The liver is subjected to NMP-L following a period of static cold storage. Organs metabolising lactate to ≤2.5 mmol/L within 4 hours of the perfusion commencing in combination with two or more of the following parameters-bile production, metabolism of glucose, a hepatic arterial flow rate ≥150 mL/min and a portal venous flow rate ≥500 mL/min, a pH ≥7.30 and/or maintain a homogeneous perfusion-will be considered viable and transplanted into a suitable consented recipient. The coprimary outcome measures are the success rate of NMP-L to produce a transplantable organ and 90-day patient post-transplant survival.
Ethics and dissemination: The protocol was approved by the National Research Ethics Service (London-Dulwich Research Ethics Committee, 16/LO/1056), the Medicines and Healthcare Products Regulatory Agency and is endorsed by the National Health Service Blood and Transplant Research, Innovation and Novel Technologies Advisory Group. The findings of this trial will be disseminated through national and international presentations and peer-reviewed publications.
Trial registration number: NCT02740608; Pre-results.
Keywords: hepatobiliary disease; hepatobiliary tumours; hepatology; transplant surgery.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: RWL and YB receive salary as Wellcome Trust research fellows.
Figures
References
-
- Williams R, Aspinall R, Bellis M, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014;384:1953–97. 10.1016/S0140-6736(14)61838-9 - DOI - PubMed
-
- NHS Blood and Transplant. Organ Donation and Transplantation Activity Report 2015/2016;16.
-
- NHS Blood and Transplant. Annual report on liver transplanation 2015/2016;16.
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials