Early low-energy versus high-energy enteral nutrition support in patients with traumatic intracerebral haemorrhage: protocol for a randomised controlled trial
- PMID: 29183931
- PMCID: PMC5719322
- DOI: 10.1136/bmjopen-2017-019199
Early low-energy versus high-energy enteral nutrition support in patients with traumatic intracerebral haemorrhage: protocol for a randomised controlled trial
Abstract
Background: Early enteral nutrition (EN) is associated with shorter hospital stay and lower infection and mortality rates in patients with intracerebral haemorrhage. However, high-energy support always causes clinical complications, such as diarrhoea and aspiration pneumonia, and the true benefit of high-energy support in these patients has not been investigated. The appropriate amount of energy support still needs further investigation. Therefore, we are performing a randomised controlled trial to investigate whether early low-energy EN can decrease mortality and feeding-related complications and improve neurological outcomes as compared with high-energy EN in traumatic intracerebral haemorrhage (TICH) patients.
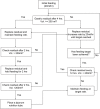
Methods/analysis: This is a randomised, single-blind clinical trial performed in one teaching hospital. 220 TICH patients will be randomly allocated to one of two groups in a 1:1 ratio: an intervention group, and a control group. The intervention group will receive early low-energy EN (10 kcal/kg/day) and the control group will receive high-energy EN (25 kcal/kg/day) for 7 days. All these patients will be followed up for 90 days. The primary outcome is all-cause 90-day mortality. Secondary outcomes include the modified Rankin score, Glasgow Outcome Scale (GOS) and the National Institutes of Health Stroke Scale (NIHSS). Outcomes will be assessed at admission, 7, 30 and 90 days after onset of this trial. The safety of EN strategies will be assessed every day during hospitalisation.
Ethics and dissemination: The trial will be conducted in accordance with the Declaration of Helsinki and has been approved by the ethics committee of Dongyang People's Hospital. The findings will be published in peer-reviewed medical journals.
Trial registration number: ChiCTR-INR-17011384; Pre-results.
Keywords: anaesthesia in neurology; nutritional support.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Surgical Trial In Traumatic intraCerebral Haemorrhage (STITCH): a randomised controlled trial of Early Surgery compared with Initial Conservative Treatment.Health Technol Assess. 2015 Sep;19(70):1-138. doi: 10.3310/hta19700. Health Technol Assess. 2015. PMID: 26346805 Free PMC article. Clinical Trial.
-
High versus low energy administration in the early phase of acute pancreatitis (GOULASH trial): protocol of a multicentre randomised double-blind clinical trial.BMJ Open. 2017 Sep 14;7(9):e015874. doi: 10.1136/bmjopen-2017-015874. BMJ Open. 2017. PMID: 28912191 Free PMC article. Clinical Trial.
-
Increased Efficacy and Safety of Enteral Nutrition Support with a Protocol (ASNET) in Noncritical Patients: A Randomized Controlled Trial.J Acad Nutr Diet. 2018 Jan;118(1):52-61. doi: 10.1016/j.jand.2017.09.020. J Acad Nutr Diet. 2018. PMID: 29274643 Clinical Trial.
-
The impact of implementation of an enteral feeding protocol on the improvement of enteral nutrition in critically ill adults.Asia Pac J Clin Nutr. 2017 Jan;26(1):27-35. doi: 10.6133/apjcn.122015.01. Asia Pac J Clin Nutr. 2017. PMID: 28049258 Review.
-
Preliminary evidence for a medical nutrition therapy protocol: enteral feedings for critically ill patients.J Am Diet Assoc. 2006 Aug;106(8):1226-41. doi: 10.1016/j.jada.2006.05.320. J Am Diet Assoc. 2006. PMID: 16863719 Review.
Cited by
-
Optimal Nutritional Factors Influencing the Duration of Mechanical Ventilation Among Adult Patients with Critical Illnesses in an Intensive Care Unit.J Multidiscip Healthc. 2021 Jun 10;14:1385-1393. doi: 10.2147/JMDH.S319553. eCollection 2021. J Multidiscip Healthc. 2021. PMID: 34140776 Free PMC article.
References
-
- Young B, Ott L, Yingling B, et al. . Nutrition and brain injury. J Neurotrauma 1992;9:S375–83. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials