Mechanical regulation of musculoskeletal system development
- PMID: 29183940
- PMCID: PMC6514418
- DOI: 10.1242/dev.151266
Mechanical regulation of musculoskeletal system development
Abstract
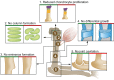
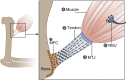
During embryogenesis, the musculoskeletal system develops while containing within itself a force generator in the form of the musculature. This generator becomes functional relatively early in development, exerting an increasing mechanical load on neighboring tissues as development proceeds. A growing body of evidence indicates that such mechanical forces can be translated into signals that combine with the genetic program of organogenesis. This unique situation presents both a major challenge and an opportunity to the other tissues of the musculoskeletal system, namely bones, joints, tendons, ligaments and the tissues connecting them. Here, we summarize the involvement of muscle-induced mechanical forces in the development of various vertebrate musculoskeletal components and their integration into one functional unit.
Keywords: Mechanoregulation; Mechanotransduction; Musculoskeletal development.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
-
- Bastow E. R., Lamb K. J., Lewthwaite J. C., Osborne A. C., Kavanagh E., Wheeler-Jones C. P. D. and Pitsillides A. A. (2005). Selective activation of the MEK-ERK pathway is regulated by mechanical stimuli in forming joints and promotes pericellular matrix formation. J. Biol. Chem. 280, 11749-11758. 10.1074/jbc.M414495200 - DOI - PubMed
-
- Bekoff A. (1981). Embryonic development of chick motor behaviour. Trends Neurosci. 4, 181-184. 10.1016/0166-2236(81)90059-X - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

