A guanine nucleotide exchange factor (GEF) limits Rab GTPase-driven membrane fusion
- PMID: 29184002
- PMCID: PMC5767875
- DOI: 10.1074/jbc.M117.812941
A guanine nucleotide exchange factor (GEF) limits Rab GTPase-driven membrane fusion
Abstract
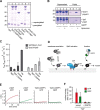
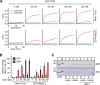
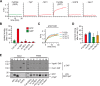
The identity of organelles in the endomembrane system of any eukaryotic cell critically depends on the correctly localized Rab GTPase, which binds effectors and thus promotes membrane remodeling or fusion. However, it is still unresolved which factors are required and therefore define the localization of the correct fusion machinery. Using SNARE-decorated proteoliposomes that cannot fuse on their own, we now demonstrate that full fusion activity can be achieved by just four soluble factors: a soluble SNARE (Vam7), a guanine nucleotide exchange factor (GEF, Mon1-Ccz1), a Rab-GDP dissociation inhibitor (GDI) complex (prenylated Ypt7-GDI), and a Rab effector complex (HOPS). Our findings reveal that the GEF Mon1-Ccz1 is necessary and sufficient for stabilizing prenylated Ypt7 on membranes. HOPS binding to Ypt7-GTP then drives SNARE-mediated fusion, which is fully GTP-dependent. We conclude that an entire fusion cascade can be controlled by a GEF.
Keywords: GDI; Mon1-Ccz1, Ypt7, Rab7, GDI; Rab; endosome; guanine nucleotide exchange factor (GEF); lysosome; membrane fusion.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Similar articles
-
The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7.Curr Biol. 2010 Sep 28;20(18):1654-9. doi: 10.1016/j.cub.2010.08.002. Curr Biol. 2010. PMID: 20797862
-
The Mon1-Ccz1 GEF activates the Rab7 GTPase Ypt7 via a longin-fold-Rab interface and association with PI3P-positive membranes.J Cell Sci. 2014 Mar 1;127(Pt 5):1043-51. doi: 10.1242/jcs.140921. Epub 2014 Jan 10. J Cell Sci. 2014. PMID: 24413168 Free PMC article.
-
Guanine nucleotide exchange factors (GEFs) have a critical but not exclusive role in organelle localization of Rab GTPases.J Biol Chem. 2013 Oct 4;288(40):28704-12. doi: 10.1074/jbc.M113.488213. Epub 2013 Aug 26. J Biol Chem. 2013. PMID: 23979137 Free PMC article.
-
Who's in control? Principles of Rab GTPase activation in endolysosomal membrane trafficking and beyond.J Cell Biol. 2021 Sep 6;220(9):e202105120. doi: 10.1083/jcb.202105120. Epub 2021 Aug 12. J Cell Biol. 2021. PMID: 34383013 Free PMC article. Review.
-
Molecular control of Rab activity by GEFs, GAPs and GDI.Small GTPases. 2018 Mar 4;9(1-2):5-21. doi: 10.1080/21541248.2016.1276999. Epub 2017 Feb 1. Small GTPases. 2018. PMID: 28055292 Free PMC article. Review.
Cited by
-
Nanoscopic anatomy of dynamic multi-protein complexes at membranes resolved by graphene-induced energy transfer.Elife. 2021 Jan 29;10:e62501. doi: 10.7554/eLife.62501. Elife. 2021. PMID: 33513092 Free PMC article.
-
A trimeric Rab7 GEF controls NPC1-dependent lysosomal cholesterol export.Nat Commun. 2020 Nov 3;11(1):5559. doi: 10.1038/s41467-020-19032-0. Nat Commun. 2020. PMID: 33144569 Free PMC article.
-
SKIP-HOPS recruits TBC1D15 for a Rab7-to-Arl8b identity switch to control late endosome transport.EMBO J. 2020 Mar 16;39(6):e102301. doi: 10.15252/embj.2019102301. Epub 2020 Feb 21. EMBO J. 2020. PMID: 32080880 Free PMC article.
-
Stochastic activation and bistability in a Rab GTPase regulatory network.Proc Natl Acad Sci U S A. 2020 Mar 24;117(12):6540-6549. doi: 10.1073/pnas.1921027117. Epub 2020 Mar 11. Proc Natl Acad Sci U S A. 2020. PMID: 32161136 Free PMC article.
-
The autophagic protein FYCO1 controls TNFRSF10/TRAIL receptor induced apoptosis and is inactivated by CASP8 (caspase 8).Autophagy. 2023 Oct;19(10):2733-2751. doi: 10.1080/15548627.2023.2229656. Epub 2023 Jul 7. Autophagy. 2023. PMID: 37418591 Free PMC article.
References
-
- Rak A., Pylypenko O., Niculae A., Pyatkov K., Goody R. S., and Alexandrov K. (2004) Structure of the Rab7:REP-1 complex: insights into the mechanism of Rab prenylation and choroideremia disease. Br. J. Anaesth. 117, 749–760 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

