Activation of planarian TRPA1 by reactive oxygen species reveals a conserved mechanism for animal nociception
- PMID: 29184198
- PMCID: PMC5856474
- DOI: 10.1038/s41593-017-0005-0
Activation of planarian TRPA1 by reactive oxygen species reveals a conserved mechanism for animal nociception
Abstract
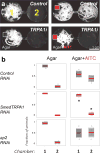
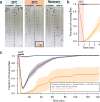
All animals must detect noxious stimuli to initiate protective behavior, but the evolutionary origin of nociceptive systems is not well understood. Here we show that noxious heat and irritant chemicals elicit robust escape behaviors in the planarian Schmidtea mediterranea and that the conserved ion channel TRPA1 is required for these responses. TRPA1-mutant Drosophila flies are also defective in noxious-heat responses. We find that either planarian or human TRPA1 can restore noxious-heat avoidance to TRPA1-mutant Drosophila, although neither is directly activated by heat. Instead, our data suggest that TRPA1 activation is mediated by H2O2 and reactive oxygen species, early markers of tissue damage rapidly produced as a result of heat exposure. Together, our data reveal a core function for TRPA1 in noxious heat transduction, demonstrate its conservation from planarians to humans, and imply that animal nociceptive systems may share a common ancestry, tracing back to a progenitor that lived more than 500 million years ago.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Bandell M, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

