Drug delivery to solid tumors: the predictive value of the multicellular tumor spheroid model for nanomedicine screening
- PMID: 29184400
- PMCID: PMC5673046
- DOI: 10.2147/IJN.S146927
Drug delivery to solid tumors: the predictive value of the multicellular tumor spheroid model for nanomedicine screening
Abstract
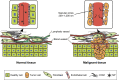
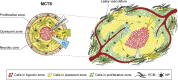
The increasing number of publications on the subject shows that nanomedicine is an attractive field for investigations aiming to considerably improve anticancer chemotherapy. Based on selective tumor targeting while sparing healthy tissue, carrier-mediated drug delivery has been expected to provide significant benefits to patients. However, despite reduced systemic toxicity, most nanodrugs approved for clinical use have been less effective than previously anticipated. The gap between experimental results and clinical outcomes demonstrates the necessity to perform comprehensive drug screening by using powerful preclinical models. In this context, in vitro three-dimensional models can provide key information on drug behavior inside the tumor tissue. The multicellular tumor spheroid (MCTS) model closely mimics a small avascular tumor with the presence of proliferative cells surrounding quiescent cells and a necrotic core. Oxygen, pH and nutrient gradients are similar to those of solid tumor. Furthermore, extracellular matrix (ECM) components and stromal cells can be embedded in the most sophisticated spheroid design. All these elements together with the physicochemical properties of nanoparticles (NPs) play a key role in drug transport, and therefore, the MCTS model is appropriate to assess the ability of NP to penetrate the tumor tissue. This review presents recent developments in MCTS models for a better comprehension of the interactions between NPs and tumor components that affect tumor drug delivery. MCTS is particularly suitable for the high-throughput screening of new nanodrugs.
Keywords: accumulation; cytotoxicity; distribution; nanodrug; tridimensional model.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Duncan R, Gaspar R. Nanomedicine(s) under the microscope. Mol Pharm. 2011;8(6):2101–2141. - PubMed
-
- Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedecine in cancer therapy: challenges, opportunities and clinical applications. J Control Release. 2015;200:138–157. - PubMed
-
- Marchal S, Hor AE, Millard M, Gillon V, Bezdetnaya L. Anticancer drug delivery: an update on clinically applied nanotherapeutics. Drugs. 2015;75(14):1601–1611. - PubMed
-
- Netti P, Berk D, Swartz M, Grodzinsky A, Jain R. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60(9):2497–2503. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

