Update on the clinical utility of an RNA interference-based treatment: focus on Patisiran
- PMID: 29184431
- PMCID: PMC5689029
- DOI: 10.2147/PGPM.S87945
Update on the clinical utility of an RNA interference-based treatment: focus on Patisiran
Abstract
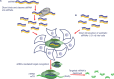
RNA interference (RNAi) is a naturally existing endogenous mechanism for post-transcriptional gene regulation, nowadays commonly utilized for functional characterization of genes and development of potential treatment strategies for diseases. RNAi-based studies for therapy, after being examined for over a decade, are finally in the pipeline for developing a potential treatment for the mutated transthyretin (TTR) gene, which gives rise to a dysfunctional TTR protein. This dysfunctional protein causes TTR amyloidosis (ATTR), an inherited, progressively incapacitating, and often fatal genetic disorder. TTR is a protein produced in the liver, and functions as a carrier for retinol-binding protein and also thyroxine. This protein facilitates the transport of vitamin A around the human body. A mutation or misprint in the code of this protein results in an abnormal folding of the protein. Therefore, not only does the transportation of the vitamin A become disabled, but also there will be formation of clusters called amyloid deposits, which attack the heart and the nerves causing some patients to be unconditionally bound to bed. ATTR is a hereditary autosomal dominant disease with a 50% chance of inheritance by offspring, even with just one of the parents having a single defective allele of this gene. Alnylam Pharmaceuticals worked on the concept of RNAi therapy for years, which led to the introduction of lipid nanoparticles encircling small interfering RNAs. The drug showed extremely positive results since the first trial, and a great percentage of defective protein reduction. This drug was later named Patisiran.
Keywords: Patisiran; RNAi; TTR amyloidosis; TTR gene; gene silencing; siRNA.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Tuzmen S, Tuzmen P, Arora S, Mousses S. RNAi-based functional pharmacogenomics. Methods Mol Biol. 2011;700:271–290. - PubMed
-
- Elbashir SM, Harborth J, Weber K, Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26(2):199–213. - PubMed
-
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. - PubMed
-
- Hannon GJ. RNA interference. Nature. 2002;418(6894):244–251. - PubMed
-
- Huppi K, Martin SE, Caplen NJ. Defining and assaying RNAi in mammalian cells. Mol Cell. 2005;17(1):1–10. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

