Development and validation of a panel of five proteins as blood biomarkers for early detection of colorectal cancer
- PMID: 29184444
- PMCID: PMC5672848
- DOI: 10.2147/CLEP.S144171
Development and validation of a panel of five proteins as blood biomarkers for early detection of colorectal cancer
Abstract
Objective: Reliable noninvasive biomarkers for early detection of colorectal cancer (CRC) are highly desirable for efficient population-based screening with high adherence rates. We aimed to discover and validate blood-based protein markers for the early detection of CRC.
Patients and methods: A two-stage design with a discovery and a validation set was used. In the discovery phase, plasma levels of 92 protein markers and serum levels of TP53 autoantibody were measured in 226 clinically recruited CRC patients and 118 controls who were free of colorectal neoplasms at screening colonoscopy. An algorithm predicting the presence of CRC was derived by Lasso regression and validated in a validation set consisting of all available 41 patients with CRC and a representative sample of 106 participants with advanced adenomas and 107 controls free of neoplasm from a large screening colonoscopy cohort (N=6018). Receiver operating characteristic (ROC) analyses were conducted to evaluate the diagnostic performance of individual biomarkers and biomarker combinations.
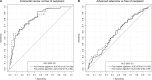
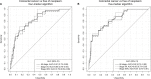
Results: An algorithm based on growth differentiation factor 15 (GDF-15), amphiregulin (AREG), Fas antigen ligand (FasL), Fms-related tyrosine kinase 3 ligand (Flt3L) and TP53 autoantibody was constructed. In the validation set, the areas under the curves of this five-marker algorithm were 0.82 (95% CI, 0.74-0.90) for detecting CRC and 0.60 (95% CI, 0.52-0.69) for detecting advanced adenomas. At cutoffs yielding 90% specificity, the sensitivities (95% CI) for detecting CRC and advanced adenomas were 56.4% (38.4%-71.8%) and 22.0% (13.4%-35.4%), respectively. The five-marker panel showed similar diagnostic efficacy for the detection of early- and late-stage CRC.
Conclusion: The identified most promising biomarkers could contribute to the development of powerful blood-based tests for CRC screening in the future.
Keywords: adenoma; amphiregulin; colorectal cancer; growth differentiation factor 15; screening.
Conflict of interest statement
Disclosure Hermann Brenner and Hongda Chen have applied for a patent “Biomarker panel for diagnosing cancer.” The other authors report no conflicts of interest in this work.
Figures
Similar articles
-
Evaluation and Validation of Plasma Proteins Using Two Different Protein Detection Methods for Early Detection of Colorectal Cancer.Cancers (Basel). 2019 Sep 25;11(10):1426. doi: 10.3390/cancers11101426. Cancers (Basel). 2019. PMID: 31557860 Free PMC article.
-
Multiplex quantitation of 270 plasma protein markers to identify a signature for early detection of colorectal cancer.Eur J Cancer. 2020 Mar;127:30-40. doi: 10.1016/j.ejca.2019.11.021. Epub 2020 Jan 21. Eur J Cancer. 2020. PMID: 31972396
-
Prospective evaluation of 64 serum autoantibodies as biomarkers for early detection of colorectal cancer in a true screening setting.Oncotarget. 2016 Mar 29;7(13):16420-32. doi: 10.18632/oncotarget.7500. Oncotarget. 2016. PMID: 26909861 Free PMC article.
-
Noninvasive fecal testing for colorectal cancer.Clin Chim Acta. 2022 Jan 1;524:123-131. doi: 10.1016/j.cca.2021.10.030. Epub 2021 Oct 28. Clin Chim Acta. 2022. PMID: 34756863 Review.
-
Fecal DNA methylation markers for detecting stages of colorectal cancer and its precursors: a systematic review.Clin Epigenetics. 2020 Aug 10;12(1):122. doi: 10.1186/s13148-020-00904-7. Clin Epigenetics. 2020. PMID: 32778176 Free PMC article.
Cited by
-
Towards Novel Non-Invasive Colorectal Cancer Screening Methods: A Comprehensive Review.Cancers (Basel). 2021 Apr 10;13(8):1820. doi: 10.3390/cancers13081820. Cancers (Basel). 2021. PMID: 33920293 Free PMC article. Review.
-
Trefoil factor(s) and CA19.9: A promising panel for early detection of pancreatic cancer.EBioMedicine. 2019 Apr;42:375-385. doi: 10.1016/j.ebiom.2019.03.056. Epub 2019 Apr 5. EBioMedicine. 2019. PMID: 30956167 Free PMC article.
-
Development of blood-based biomarker tests for early detection of colorectal neoplasia: Influence of blood collection timing and handling procedures.Clin Chim Acta. 2020 Aug;507:39-53. doi: 10.1016/j.cca.2020.03.035. Epub 2020 Apr 6. Clin Chim Acta. 2020. PMID: 32272156 Free PMC article.
-
A two-tiered targeted proteomics approach to identify pre-diagnostic biomarkers of colorectal cancer risk.Sci Rep. 2021 Mar 4;11(1):5151. doi: 10.1038/s41598-021-83968-6. Sci Rep. 2021. PMID: 33664295 Free PMC article.
-
Unveiling the protein landscape for early detection of colorectal precancerous lesions.Clin Proteomics. 2025 Aug 21;22(1):27. doi: 10.1186/s12014-025-09552-6. Clin Proteomics. 2025. PMID: 40841878 Free PMC article.
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. - PubMed
-
- Zorzi M, Fedeli U, Schievano E, et al. Impact on colorectal cancer mortality of screening programmes based on the faecal immunochemical test. Gut. 2015;64(5):784–790. - PubMed
-
- Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541–1549. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

