The Role of Celestial Compass Information in Cataglyphis Ants during Learning Walks and for Neuroplasticity in the Central Complex and Mushroom Bodies
- PMID: 29184487
- PMCID: PMC5694495
- DOI: 10.3389/fnbeh.2017.00226
The Role of Celestial Compass Information in Cataglyphis Ants during Learning Walks and for Neuroplasticity in the Central Complex and Mushroom Bodies
Abstract
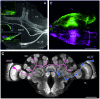
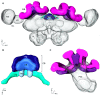
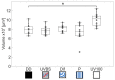
Central place foragers are faced with the challenge to learn the position of their nest entrance in its surroundings, in order to find their way back home every time they go out to search for food. To acquire navigational information at the beginning of their foraging career, Cataglyphis noda performs learning walks during the transition from interior worker to forager. These small loops around the nest entrance are repeatedly interrupted by strikingly accurate back turns during which the ants stop and precisely gaze back to the nest entrance-presumably to learn the landmark panorama of the nest surroundings. However, as at this point the complete navigational toolkit is not yet available, the ants are in need of a reference system for the compass component of the path integrator to align their nest entrance-directed gazes. In order to find this directional reference system, we systematically manipulated the skylight information received by ants during learning walks in their natural habitat, as it has been previously suggested that the celestial compass, as part of the path integrator, might provide such a reference system. High-speed video analyses of distinct learning walk elements revealed that even exclusion from the skylight polarization pattern, UV-light spectrum and the position of the sun did not alter the accuracy of the look back to the nest behavior. We therefore conclude that C. noda uses a different reference system to initially align their gaze directions. However, a comparison of neuroanatomical changes in the central complex and the mushroom bodies before and after learning walks revealed that exposure to UV light together with a naturally changing polarization pattern was essential to induce neuroplasticity in these high-order sensory integration centers of the ant brain. This suggests a crucial role of celestial information, in particular a changing polarization pattern, in initially calibrating the celestial compass system.
Keywords: central complex; desert ants; look-back behavior; memory; mushroom body; sky-compass pathway; vector navigation; visual orientation.
Figures



References
-
- Anderson J. B., Vander Meer R. K. (1993). Magnetic orientation in the fire ant, Solenopsis invicta. Naturwissenschaften 80, 568–570. 10.1007/bf01149274 - DOI
-
- Banks A. N., Srygley R. B. (2003). Orientation by magnetic field in leaf-cutter ants, Atta colombica (Hymenoptera: Formicidae). Ethology 109, 835–846. 10.1046/j.0179-1613.2003.00927.x - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

