Polygalae Radix Extract Prevents Axonal Degeneration and Memory Deficits in a Transgenic Mouse Model of Alzheimer's Disease
- PMID: 29184495
- PMCID: PMC5694549
- DOI: 10.3389/fphar.2017.00805
Polygalae Radix Extract Prevents Axonal Degeneration and Memory Deficits in a Transgenic Mouse Model of Alzheimer's Disease
Abstract
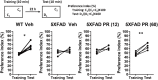
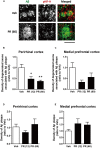
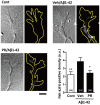
Memory impairments in Alzheimer's disease (AD) occur due to degenerated axons and disrupted neural networks. Since only limited recovery is possible after the destruction of neural networks, preventing axonal degeneration during the early stages of disease progression is necessary to prevent AD. Polygalae Radix (roots of Polygala tenuifolia; PR) is a traditional herbal medicine used for sedation and amnesia. In this study, we aimed to clarify and analyze the preventive effects of PR against memory deficits in a transgenic AD mouse model, 5XFAD. 5XFAD mice demonstrated memory deficits at the age of 5 months. Thus, the water extract of Polygalae Radix (PR extract) was orally administered to 4-month-old 5XFAD mice that did not show signs of memory impairment. After consecutive administrations for 56 days, the PR extract prevented cognitive deficit and axon degeneration associated with the accumulation of amyloid β (Aβ) plaques in the perirhinal cortex of the 5XFAD mice. PR extract did not influence the formation of Aβ plaques in the brain of the 5XFAD mice. In cultured neurons, the PR extract prevented axonal growth cone collapse and axonal atrophy induced by Aβ. Additionally, it prevented Aβ-induced endocytosis at the growth cone of cultured neurons. Our previous study reported that endocytosis inhibition was enough to prevent Aβ-induced growth cone collapse, axonal degeneration, and memory impairments. Therefore, the PR extract possibly prevented axonal degeneration and memory impairment by inhibiting endocytosis. PR is the first preventive drug candidate for AD that inhibits endocytosis in neurons.
Keywords: 5XFAD mice; Alzheimer’s disease; Polygalae Radix; amyloid β; axon degeneration; endocytosis; growth cone collapse.
Figures



References
-
- Chinese Pharmacopoeia Commission (2015). “Polygalae radix,” in Pharmacopoeia of the People’s Republic of China eds Zhao Y., Fan Z., Huang K., Li Z., Gao Y. (Beijing: People’s Medicine Publishing House Press; ).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

