IL-17 and TNF-α Are Key Mediators of Moraxella catarrhalis Triggered Exacerbation of Allergic Airway Inflammation
- PMID: 29184554
- PMCID: PMC5694487
- DOI: 10.3389/fimmu.2017.01562
IL-17 and TNF-α Are Key Mediators of Moraxella catarrhalis Triggered Exacerbation of Allergic Airway Inflammation
Abstract
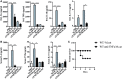
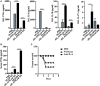
Alterations of the airway microbiome are often associated with pulmonary diseases. For example, detection of the bacterial pathogen Moraxella catarrhalis in the upper airways is linked with an increased risk to develop or exacerbate asthma. However, the mechanisms by which M. catarrhalis augments allergic airway inflammation (AAI) remain unclear. We here characterized the cellular and soluble mediators of M. catarrhalis triggered excacerbation of AAI in wt and IL-17 deficient as well as in animals treated with TNF-α and IL-6 neutralizing antibodies. We compared the type of inflammatory response in M. catarrhalis infected, house dust mite (HDM)-allergic and animals infected with M. catarrhalis at different time points of HDM sensitization. We found that airway infection of mice with M. catarrhalis triggers a strong inflammatory response with massive neutrophilic infiltrates, high amounts of IL-6 and TNF-α and moderate levels of CD4+ T-cell-derived IFN-γ and IL-17. If bacterial infection occurred during HDM allergen sensitization, the allergic airway response was exacerbated, particularly by the expansion of Th17 cells and increased TNF-α levels. Neutralization of IL-17 or TNF-α but not IL-6 resulted in accelerated clearance of M. catarrhalis and effectively prevented infection-induced exacerbation of AAI. Taken together, our data demonstrate an essential role for TNF-α and IL-17 in infection-triggered exacerbation of AAI.
Keywords: IL-17; Moraxellaceae infections; TNF-α; exacerbation of allergic reactions; exacerbation of pulmonary inflammation; infection and allergy; microbial exacerbation of pulmonary inflammation; pulmonary inflammation.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

