Targeting DNA Replication and Repair for the Development of Novel Therapeutics against Tuberculosis
- PMID: 29184888
- PMCID: PMC5694481
- DOI: 10.3389/fmolb.2017.00075
Targeting DNA Replication and Repair for the Development of Novel Therapeutics against Tuberculosis
Abstract
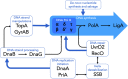
Mycobacterium tuberculosis is the etiological agent of tuberculosis (TB), an infectious disease which results in approximately 10 million incident cases and 1.4 million deaths globally each year, making it the leading cause of mortality from infection. An effective frontline combination chemotherapy exists for TB; however, this regimen requires the administration of four drugs in a 2 month long intensive phase followed by a continuation phase of a further 4 months with two of the original drugs, and is only effective for the treatment of drug-sensitive TB. The emergence and global spread of multidrug-resistant (MDR) as well as extensively drug-resistant (XDR) strains of M. tuberculosis, and the complications posed by co-infection with the human immunodeficiency virus (HIV) and other co-morbidities such as diabetes, have prompted urgent efforts to develop shorter regimens comprising new compounds with novel mechanisms of action. This demands that researchers re-visit cellular pathways and functions that are essential to M. tuberculosis survival and replication in the host but which are inadequately represented amongst the targets of current anti-mycobacterial agents. Here, we consider the DNA replication and repair machinery as a source of new targets for anti-TB drug development. Like most bacteria, M. tuberculosis encodes a complex array of proteins which ensure faithful and accurate replication and repair of the chromosomal DNA. Many of these are essential; so, too, are enzymes in the ancillary pathways of nucleotide biosynthesis, salvage, and re-cycling, suggesting the potential to inhibit replication and repair functions at multiple stages. To this end, we provide an update on the state of chemotherapeutic inhibition of DNA synthesis and related pathways in M. tuberculosis. Given the established links between genotoxicity and mutagenesis, we also consider the potential implications of targeting DNA metabolic pathways implicated in the development of drug resistance in M. tuberculosis, an organism which is unusual in relying exclusively on de novo mutations and chromosomal rearrangements for evolution, including the acquisition of drug resistance. In that context, we conclude by discussing the feasibility of targeting mutagenic pathways in an ancillary, "anti-evolution" strategy aimed at protecting existing and future TB drugs.
Keywords: DNA replication; Tuberculosis; bacteria; drug resistance; drug targets.
Figures
Similar articles
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
-
[Recent progress in mycobacteriology].Kekkaku. 2007 Oct;82(10):783-99. Kekkaku. 2007. PMID: 18018602 Japanese.
-
[Frontier of mycobacterium research--host vs. mycobacterium].Kekkaku. 2005 Sep;80(9):613-29. Kekkaku. 2005. PMID: 16245793 Japanese.
-
API TB Consensus Guidelines 2006: Management of pulmonary tuberculosis, extra-pulmonary tuberculosis and tuberculosis in special situations.J Assoc Physicians India. 2006 Mar;54:219-34. J Assoc Physicians India. 2006. PMID: 16800350
-
The resumption of consumption -- a review on tuberculosis.Mem Inst Oswaldo Cruz. 2006 Nov;101(7):697-714. doi: 10.1590/s0074-02762006000700001. Mem Inst Oswaldo Cruz. 2006. PMID: 17160276 Review.
Cited by
-
The War against Tuberculosis: A Review of Natural Compounds and Their Derivatives.Molecules. 2020 Jun 30;25(13):3011. doi: 10.3390/molecules25133011. Molecules. 2020. PMID: 32630150 Free PMC article. Review.
-
High-Throughput Exonuclease Assay Based on the Fluorescent Base Analogue 2-Aminopurine.ACS Omega. 2023 Feb 20;8(9):8285-8292. doi: 10.1021/acsomega.2c06577. eCollection 2023 Mar 7. ACS Omega. 2023. PMID: 36910963 Free PMC article.
-
Targeting Genome Integrity in Mycobacterium Tuberculosis: From Nucleotide Synthesis to DNA Replication and Repair.Molecules. 2020 Mar 7;25(5):1205. doi: 10.3390/molecules25051205. Molecules. 2020. PMID: 32156001 Free PMC article. Review.
-
In-vivo studies on Transitmycin, a potent Mycobacterium tuberculosis inhibitor.PLoS One. 2023 Mar 3;18(3):e0282454. doi: 10.1371/journal.pone.0282454. eCollection 2023. PLoS One. 2023. PMID: 36867599 Free PMC article.
-
Mycobacterium tuberculosis Pathogenesis, Infection Prevention and Treatment.Pathogens. 2020 May 18;9(5):385. doi: 10.3390/pathogens9050385. Pathogens. 2020. PMID: 32443469 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

