Evaluating and improving recruitment and retention in an mHealth clinical trial: an example of iterating methods during a trial
- PMID: 29184901
- PMCID: PMC5682361
- DOI: 10.21037/mhealth.2017.09.02
Evaluating and improving recruitment and retention in an mHealth clinical trial: an example of iterating methods during a trial
Abstract
Background: Recruitment and retention strategy investigations in mHealth clinical trials are rare. Technology presents an opportunity to intensely and remotely evaluate recruitment, use of mobile apps, and retention, leading to new insights for continuous improvement of mHealth trials. The objective of this paper is to present a case study in which a trial evaluated and changed strategies during a clinical trial to improve recruitment, adherence to study protocols, and retention in the mHealth trial.
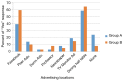
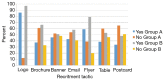
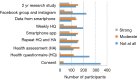
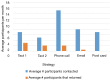
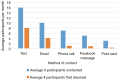
Methods: In Fall 2015, the NUYou trial enrolled 150 college freshmen in an mHealth protocol. Three months after study initiation, NUYou struggled to meet recruitment goals and maintain anticipated usage levels of the study smartphone application. Two sets of data were collected to improve recruitment and retention: a survey about recruitment was sent to the target population and surveys regarding usability of the app was sent to the study sample. Survey results informed improvements in recruitment strategies, the study retention protocol, and the smartphone application.
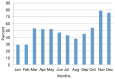
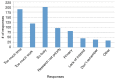
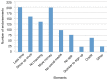
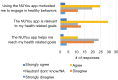
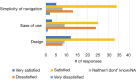
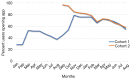
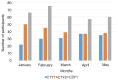
Results: Survey results revealed several insights including misunderstanding components of the trial by potential participants, low perceived usefulness of the app, and little recall or impact of the incentive structure. After implementation of user-centered improvements, the second cohort of NUYou recruitment in the fall of 2016 produced an equal sample size in 4 weeks less time. Winter quarter of 2016 compared to 2017 demonstrated an improvement in retention via app use and completion of weekly in-app surveys.
Conclusions: Recruitment and retention in clinical trials continues to be a critical challenge and mHealth trials may present both unique challenges and opportunities. To our knowledge, this is the first study to describe a systematic evaluation followed by changes and further evaluation to recruitment, use of the mHealth application, adherence to study protocol, and retention during an mHealth clinical trial. Future work should adopt and explicitly study these processes to optimize both enrollment and retention in these types of trials to preserve validity and reliability of research results.
Keywords: Research subject recruitment; clinical research protocol; incentives.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
