Systems biology of the IMIDIA biobank from organ donors and pancreatectomised patients defines a novel transcriptomic signature of islets from individuals with type 2 diabetes
- PMID: 29185012
- PMCID: PMC5803296
- DOI: 10.1007/s00125-017-4500-3
Systems biology of the IMIDIA biobank from organ donors and pancreatectomised patients defines a novel transcriptomic signature of islets from individuals with type 2 diabetes
Abstract
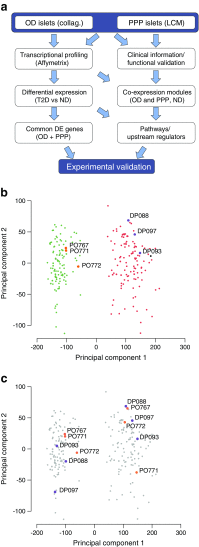
Aims/hypothesis: Pancreatic islet beta cell failure causes type 2 diabetes in humans. To identify transcriptomic changes in type 2 diabetic islets, the Innovative Medicines Initiative for Diabetes: Improving beta-cell function and identification of diagnostic biomarkers for treatment monitoring in Diabetes (IMIDIA) consortium ( www.imidia.org ) established a comprehensive, unique multicentre biobank of human islets and pancreas tissues from organ donors and metabolically phenotyped pancreatectomised patients (PPP).
Methods: Affymetrix microarrays were used to assess the islet transcriptome of islets isolated either by enzymatic digestion from 103 organ donors (OD), including 84 non-diabetic and 19 type 2 diabetic individuals, or by laser capture microdissection (LCM) from surgical specimens of 103 PPP, including 32 non-diabetic, 36 with type 2 diabetes, 15 with impaired glucose tolerance (IGT) and 20 with recent-onset diabetes (<1 year), conceivably secondary to the pancreatic disorder leading to surgery (type 3c diabetes). Bioinformatics tools were used to (1) compare the islet transcriptome of type 2 diabetic vs non-diabetic OD and PPP as well as vs IGT and type 3c diabetes within the PPP group; and (2) identify transcription factors driving gene co-expression modules correlated with insulin secretion ex vivo and glucose tolerance in vivo. Selected genes of interest were validated for their expression and function in beta cells.
Results: Comparative transcriptomic analysis identified 19 genes differentially expressed (false discovery rate ≤0.05, fold change ≥1.5) in type 2 diabetic vs non-diabetic islets from OD and PPP. Nine out of these 19 dysregulated genes were not previously reported to be dysregulated in type 2 diabetic islets. Signature genes included TMEM37, which inhibited Ca2+-influx and insulin secretion in beta cells, and ARG2 and PPP1R1A, which promoted insulin secretion. Systems biology approaches identified HNF1A, PDX1 and REST as drivers of gene co-expression modules correlated with impaired insulin secretion or glucose tolerance, and 14 out of 19 differentially expressed type 2 diabetic islet signature genes were enriched in these modules. None of these signature genes was significantly dysregulated in islets of PPP with impaired glucose tolerance or type 3c diabetes.
Conclusions/interpretation: These studies enabled the stringent definition of a novel transcriptomic signature of type 2 diabetic islets, regardless of islet source and isolation procedure. Lack of this signature in islets from PPP with IGT or type 3c diabetes indicates differences possibly due to peculiarities of these hyperglycaemic conditions and/or a role for duration and severity of hyperglycaemia. Alternatively, these transcriptomic changes capture, but may not precede, beta cell failure.
Keywords: Beta cell; Biobank; Diabetes; Gene expression; Insulin secretion; Islet; Laser capture microdissection; Organ donor; Pancreatectomy; Systems biology.
Figures



References
Publication types
MeSH terms
Grants and funding
- 155005/Innovative Medicines Initiative Joint Untertaking/International
- Advanced Investigator Award WT098424AIA/WT_/Wellcome Trust/United Kingdom
- FS/11/25/28740/BHF_/British Heart Foundation/United Kingdom
- MR/N020472/1/MRC_/Medical Research Council/United Kingdom
- MR/K023667/1/MRC_/Medical Research Council/United Kingdom
- MR/M012646/1/MRC_/Medical Research Council/United Kingdom
- MR/L02036X/1/MRC_/Medical Research Council/United Kingdom
- MR/K001981/1/MRC_/Medical Research Council/United Kingdom
- MR/J0003042/1/MRC_/Medical Research Council/United Kingdom
- MC_U120097112/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MR/R010676/1/MRC_/Medical Research Council/United Kingdom
- WT098424AIA/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

