Characterising Pre-pubertal Resistance to Death from Endotoxemia
- PMID: 29185479
- PMCID: PMC5707402
- DOI: 10.1038/s41598-017-16743-1
Characterising Pre-pubertal Resistance to Death from Endotoxemia
Abstract
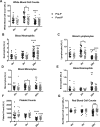
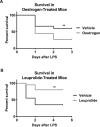
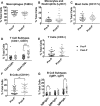
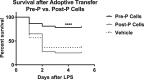
Sepsis is a common and deadly syndrome in which a dysregulated host response to infection causes organ failure and death. The current lack of treatment options suggests that a new approach to studying sepsis is needed. Pre-pubertal children show a relative resistance to death from severe infections and sepsis. To explore this phenomenon experimentally, we used an endotoxemia model of sepsis in mice. Following intra-peritoneal injection of endotoxin, pre-pubertal mice showed greater survival than post-pubertal mice (76.3% vs. 28.6%), despite exhibiting a similar degree of inflammation after two hours. Age-associated differences in the inflammatory response only became evident at twenty hours, when post-pubertal mice showed prolonged elevation of serum cytokines and differential recruitment of peritoneal immune cells. Mechanistically, prevention of puberty by hormonal blockade or acceleration of puberty by oestrogen treatment led to increased or decreased survival from endotoxemia, respectively. Additionally, the adoptive transfer of pre-pubertal peritoneal cells improved the survival of post-pubertal recipient mice, while post-pubertal peritoneal cells or vehicle did not. These data establish a model for studying childhood resistance to mortality from endotoxemia, demonstrate that oestrogen is responsible for an increased susceptibility to mortality after puberty, and identify peritoneal cells as mediators of pre-pubertal resistance.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Linder, F. E. & Grove, R. D. Vital statistics rates in the United States 1900–1940. 248, 254 (US Government Printing Office, Washington, D.C., 1947).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

