Single-Molecule Fluorescence Reveals Commonalities and Distinctions among Natural and in Vitro-Selected RNA Tertiary Motifs in a Multistep Folding Pathway
- PMID: 29185740
- PMCID: PMC5748328
- DOI: 10.1021/jacs.7b08870
Single-Molecule Fluorescence Reveals Commonalities and Distinctions among Natural and in Vitro-Selected RNA Tertiary Motifs in a Multistep Folding Pathway
Abstract
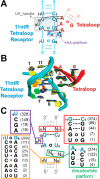
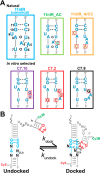
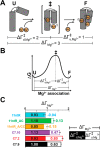
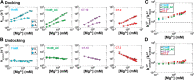
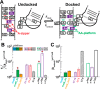
Decades of study of the RNA folding problem have revealed that diverse and complex structured RNAs are built from a common set of recurring structural motifs, leading to the perspective that a generalizable model of RNA folding may be developed from understanding of the folding properties of individual structural motifs. We used single-molecule fluorescence to dissect the kinetic and thermodynamic properties of a set of variants of a common tertiary structural motif, the tetraloop/tetraloop-receptor (TL/TLR). Our results revealed a multistep TL/TLR folding pathway in which preorganization of the ubiquitous AA-platform submotif precedes the formation of the docking transition state and tertiary A-minor hydrogen bond interactions form after the docking transition state. Differences in ion dependences between TL/TLR variants indicated the occurrence of sequence-dependent conformational rearrangements prior to and after the formation of the docking transition state. Nevertheless, varying the junction connecting the TL/TLR produced a common kinetic and ionic effect for all variants, suggesting that the global conformational search and compaction electrostatics are energetically independent from the formation of the tertiary motif contacts. We also found that in vitro-selected variants, despite their similar stability at high Mg2+ concentrations, are considerably less stable than natural variants under near-physiological ionic conditions, and the occurrence of the TL/TLR sequence variants in Nature correlates with their thermodynamic stability in isolation. Overall, our findings are consistent with modular but complex energetic properties of RNA structural motifs and will aid in the eventual quantitative description of RNA folding from its secondary and tertiary structural elements.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




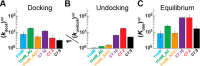
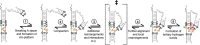

References
-
- Stagno J. R.; Liu Y.; Bhandari Y. R.; Conrad C. E.; Panja S.; Swain M.; Fan L.; Nelson G.; Li C.; Wendel D. R.; White T. A.; Coe J. D.; Wiedorn M. O.; Knoska J.; Oberthuer D.; Tuckey R. A.; Yu P.; Dyba M.; Tarasov S. G.; Weierstall U.; Grant T. D.; Schwieters C. D.; Zhang J.; Ferré-D’Amaré A. R.; Fromme P.; Draper D. E.; Liang M.; Hunter M. S.; Boutet S.; Tan K.; Zuo X.; Ji X.; Barty A.; Zatsepin N. A.; Chapman H. N.; Spence J. C. H.; Woodson S. A.; Wang Y.-X. Nature 2016, 541 (7636), 242–246. 10.1038/nature20599. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

