Desvenlafaxine Versus Placebo in the Treatment of Children and Adolescents with Major Depressive Disorder
- PMID: 29185786
- PMCID: PMC5771531
- DOI: 10.1089/cap.2017.0099
Desvenlafaxine Versus Placebo in the Treatment of Children and Adolescents with Major Depressive Disorder
Abstract
Objective: To evaluate the short-term efficacy and safety of desvenlafaxine versus placebo in the treatment of children and adolescents with major depressive disorder (MDD).
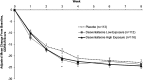
Methods: Outpatient children (7-11 years) and adolescents (12-17 years) who met DSM-IV-TR criteria for MDD and had screening and baseline Children's Depression Rating Scale-Revised (CDRS-R) total scores >40 were randomly assigned to 8 weeks of treatment with placebo, low exposure desvenlafaxine (20, 30, or 35 mg/day based on baseline weight), or higher exposure desvenlafaxine (25, 35, or 50 mg/day based on baseline weight). The primary efficacy endpoint was change from baseline in CDRS-R total score at week 8, analyzed using a mixed-effects model for repeated measures. Secondary efficacy assessments included Clinical Global Impressions-Severity and Clinical Global Impressions-Improvement scales. Safety assessments included adverse events and the Columbia-Suicide Severity Rating Scale.
Results: The safety population included 363 patients (children, n = 109; adolescents, n = 254). No statistical separation from placebo was observed for either desvenlafaxine group for CDRS-R total score or for any secondary efficacy endpoint. At week 8, adjusted mean (standard error) changes from baseline in CDRS-R total score for the desvenlafaxine low exposure, desvenlafaxine high exposure, and placebo groups were -23.7 (1.1), -24.4 (1.1), and -22.9 (1.1), respectively. The incidence of adverse events was similar among groups.
Conclusion: Low and high exposure desvenlafaxine groups did not demonstrate efficacy for the treatment of MDD in children and adolescents in this double-blind, placebo-controlled trial. Desvenlafaxine (20-50 mg/day) was generally safe and well tolerated with no new safety signals identified in pediatric patients with MDD in this study.
Trial registration: ClinicalTrials.gov NCT01371734.
Keywords: adolescents; children; clinical trial; desvenlafaxine; major depressive disorder; treatment efficacy.
Figures

References
-
- Atkinson SD, Prakash A, Zhang Q, Pangallo BA, Bangs ME, Emslie GJ, March JS: A double-blind efficacy and safety study of duloxetine flexible dosing in children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 24:180–189, 2014 - PubMed
-
- Birmaher B, Brent D, Bernet W, Bukstein O, Walter H, Benson RS, Chrisman A, Farchione T, Greenhill L, Hamilton J, Keable H, Kinlan J, Schoettle U, Stock S, Ptakowski KK, Medicus J: Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry 46:1503–1526, 2007 - PubMed
-
- Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, Perel J, Nelson B: Childhood and adolescent depression: A review of the past 10 years. Part I. J Am Acad Child Adolesc Psychiatry 35:1427–1439, 1996 - PubMed
-
- Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA: Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry 166:42–49, 2009 - PubMed
-
- Carrasco JL, Kornstein SG, McIntyre RS, Fayyad R, Prieto R, Salas M, Mackell J, Boucher M: An integrated analysis of the efficacy and safety of desvenlafaxine in the treatment of major depressive disorder. Int Clin Psychopharmacol 31:134–146, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
