Development and Validation of a Novel Integrated Clinical-Genomic Risk Group Classification for Localized Prostate Cancer
- PMID: 29185869
- PMCID: PMC6530900
- DOI: 10.1200/JCO.2017.74.2940
Development and Validation of a Novel Integrated Clinical-Genomic Risk Group Classification for Localized Prostate Cancer
Abstract
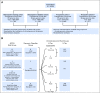
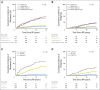
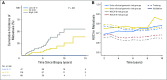
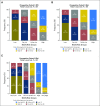
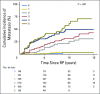
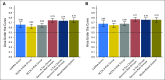
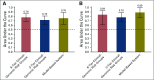
Purpose It is clinically challenging to integrate genomic-classifier results that report a numeric risk of recurrence into treatment recommendations for localized prostate cancer, which are founded in the framework of risk groups. We aimed to develop a novel clinical-genomic risk grouping system that can readily be incorporated into treatment guidelines for localized prostate cancer. Materials and Methods Two multicenter cohorts (n = 991) were used for training and validation of the clinical-genomic risk groups, and two additional cohorts (n = 5,937) were used for reclassification analyses. Competing risks analysis was used to estimate the risk of distant metastasis. Time-dependent c-indices were constructed to compare clinicopathologic risk models with the clinical-genomic risk groups. Results With a median follow-up of 8 years for patients in the training cohort, 10-year distant metastasis rates for National Comprehensive Cancer Network (NCCN) low, favorable-intermediate, unfavorable-intermediate, and high-risk were 7.3%, 9.2%, 38.0%, and 39.5%, respectively. In contrast, the three-tier clinical-genomic risk groups had 10-year distant metastasis rates of 3.5%, 29.4%, and 54.6%, for low-, intermediate-, and high-risk, respectively, which were consistent in the validation cohort (0%, 25.9%, and 55.2%, respectively). C-indices for the clinical-genomic risk grouping system (0.84; 95% CI, 0.61 to 0.93) were improved over NCCN (0.73; 95% CI, 0.60 to 0.86) and Cancer of the Prostate Risk Assessment (0.74; 95% CI, 0.65 to 0.84), and 30% of patients using NCCN low/intermediate/high would be reclassified by the new three-tier system and 67% of patients would be reclassified from NCCN six-tier (very-low- to very-high-risk) by the new six-tier system. Conclusion A commercially available genomic classifier in combination with standard clinicopathologic variables can generate a simple-to-use clinical-genomic risk grouping that more accurately identifies patients at low, intermediate, and high risk for metastasis and can be easily incorporated into current guidelines to better risk-stratify patients.
Figures

Comment in
-
Prostate cancer: Genomic information improves risk prediction.Nat Rev Clin Oncol. 2018 Feb;15(2):66-67. doi: 10.1038/nrclinonc.2017.205. Epub 2017 Dec 19. Nat Rev Clin Oncol. 2018. PMID: 29255241 No abstract available.
-
Prostate cancer: Genomic information improves risk prediction.Nat Rev Urol. 2018 Feb;15(2):68. doi: 10.1038/nrurol.2017.218. Epub 2017 Dec 19. Nat Rev Urol. 2018. PMID: 29256492 No abstract available.
-
Optimization of Risk Stratification in Localized Prostate Cancer.J Clin Oncol. 2018 Feb 20;36(6):528-532. doi: 10.1200/JCO.2017.76.2971. Epub 2018 Jan 5. J Clin Oncol. 2018. PMID: 29303624
-
Re: Development and Validation of a Novel Integrated Clinical-Genomic Risk Group Classification for Localized Prostate Cancer.J Urol. 2018 Sep;200(3):498-499. doi: 10.1016/j.juro.2018.05.116. Epub 2018 May 30. J Urol. 2018. PMID: 30412981 No abstract available.
References
-
- Mohler JL, Armstrong AJ, Bahnson RR, et al. : Prostate cancer, version 1.2016. J Natl Compr Canc Netw 14:19-30, 2016 - PubMed
-
- D’Amico AV, Whittington R, Malkowicz SB, et al. : Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280:969-974, 1998 - PubMed
-
- Amin MB, Edge S, Greene F. et al: AJCC Cancer Staging Manual (8th edition). New York, NY, Springer, 2017.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

