MUC1: The First Respiratory Mucin with an Anti-Inflammatory Function
- PMID: 29186029
- PMCID: PMC5742799
- DOI: 10.3390/jcm6120110
MUC1: The First Respiratory Mucin with an Anti-Inflammatory Function
Abstract
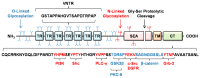
MUC1 is a membrane-bound mucin expressed on the apical surfaces of most mucosal epithelial cells. In normal lung epithelia, MUC1 is a binding site for Pseudomonas aeruginosa, an opportunistic human pathogen of great clinical importance. It has now been established that MUC1 also serves an anti-inflammatory role in the airways that is initiated late in the course of a bacterial infection and is mediated through inhibition of Toll-like receptor (TLR) signaling. MUC1 expression was initially shown to interfere with TLR5 signaling in response to P. aeruginosa flagellin, but has since been extended to other TLRs. These new findings point to an immunomodulatory role for MUC1 during P. aeruginosa lung infection, particularly during the resolution phase of inflammation. This review briefly summarizes the recent characterization of MUC1's anti-inflammatory properties in both the respiratory tract and extrapulmonary tissues.
Keywords: MUC1; Pseudomonas aeruginosa; Toll-like receptor; inflammation; lung; membrane-tethered mucin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kim K.C., Hyun S.W., Kim B.T., Meerzaman D., Lee M.K., Lillehoj E.P. Pseudomonas adhesion to MUC1 mucins: A potential role of MUC1 mucins in clearance of inhaled bacteria. In: Salathe M., editor. Cilia and Mucus: From Development to Respiratory Defense. CRC Press; Boca Raton, FL, USA: 2001. pp. 217–224.
-
- Lillehoj E.P., Hyun S.W., Kim B.T., Zhang X.G., Lee D.I., Rowland S., Kim K.C. Muc1 mucins on the cell surface are adhesion sites for Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L181–L187. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

