HPLC-PDA-MS/MS Characterization of Bioactive Secondary Metabolites from Turraea fischeri Bark Extract and Its Antioxidant and Hepatoprotective Activities In Vivo
- PMID: 29186050
- PMCID: PMC6149860
- DOI: 10.3390/molecules22122089
HPLC-PDA-MS/MS Characterization of Bioactive Secondary Metabolites from Turraea fischeri Bark Extract and Its Antioxidant and Hepatoprotective Activities In Vivo
Erratum in
-
Correction: Sobeh et al. HPLC-PDA-MS/MS Characterization of Bioactive Secondary Metabolites from Turraea fischeri Bark Extract and Its Antioxidant and Hepatoprotective Activities In Vivo. Molecules 2017, 22, 2089.Molecules. 2024 Jul 15;29(14):3319. doi: 10.3390/molecules29143319. Molecules. 2024. PMID: 39065021 Free PMC article.
Abstract
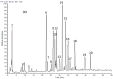
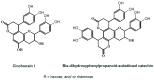
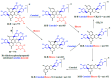
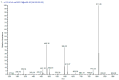
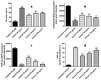
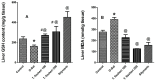
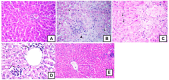
Turraea fischeri is an East African traditional herb, which is widely used in traditional medicine. In this study, we profiled the secondary metabolites in the methanol extract of T. fischeri bark using HPLC-PDA-ESI-MS/MS, and 20 compounds were tentatively identified. Several isomers of the flavonolignan cinchonain-I and bis-dihydroxyphenylpropanoid-substituted catechin hexosides dominated the extract. Robust in vitro and in vivo antioxidant properties were observed in 1,1-diphenyl-2-picrylhydrazyl radical scavenging assay (DPPH) and ferric reducing antioxidant power (FRAP) assay, and in the model organism Caenorhabditis elegans. Additionally, the extract exhibited promising hepatoprotective activities in D-galactosamine (D-GaIN) treated rats. A significant reduction in the elevated levels of aspartate aminotransferase (AST), total bilirubin, gamma-glutamyltransferase (GGT), and malondialdehyde (MDA) and increase of glutathione (GSH) was observed in rats treated with the bark extract in addition to D-galactosamine when compared with rats treated with D-galactosamine alone. In conclusion, T. fischeri is apromising candidate for health-promoting and for pharmaceutical applications.
Keywords: HPLC-PDA-ESI-MS/MS; Turraea fischeri; antioxidant; cinchonains; flavonolignan; hepatoprotection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Adly A.A. Oxidative stress and disease: An updated review. Res. J. Immunol. 2010;3:129–145.
-
- Van Wyk B.-E., Wink M. Phytomedicines, Herbal Drugs and Poisons. University of Chicago Press; Chicago, IL, USA: 2015.
-
- Van Wyk B.-E., Wink M. Medicinal Plants of the World. 2nd ed. CABI; Wallingford, UK: 2017.
-
- Mabberley D.J. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classifications and Uses. 3rd ed. Cambridge University Press; Cambridge, UK: 2008.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

