Regulation of actions and habits by ventral hippocampal trkB and adolescent corticosteroid exposure
- PMID: 29186135
- PMCID: PMC5724896
- DOI: 10.1371/journal.pbio.2003000
Regulation of actions and habits by ventral hippocampal trkB and adolescent corticosteroid exposure
Abstract
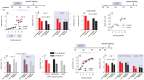
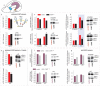
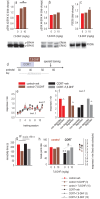
In humans and rodents, stress promotes habit-based behaviors that can interfere with action-outcome decision-making. Further, developmental stressor exposure confers long-term habit biases across rodent-primate species. Despite these homologies, mechanisms remain unclear. We first report that exposure to the primary glucocorticoid corticosterone (CORT) in adolescent mice recapitulates multiple neurobehavioral consequences of stressor exposure, including long-lasting biases towards habit-based responding in a food-reinforced operant conditioning task. In both adolescents and adults, CORT also caused a shift in the balance between full-length tyrosine kinase receptor B (trkB) and a truncated form of this neurotrophin receptor, favoring the inactive form throughout multiple corticolimbic brain regions. In adolescents, phosphorylation of the trkB substrate extracellular signal-regulated kinase 42/44 (ERK42/44) in the ventral hippocampus was also diminished, a long-term effect that persisted for at least 12 wk. Administration of the trkB agonist 7,8-dihydroxyflavone (7,8-DHF) during adolescence at doses that stimulated ERK42/44 corrected long-lasting corticosterone-induced behavioral abnormalities. Meanwhile, viral-mediated overexpression of truncated trkB in the ventral hippocampus reduced local ERK42/44 phosphorylation and was sufficient to induce habit-based and depression-like behaviors. Together, our findings indicate that ventral hippocampal trkB is essential to goal-directed action selection, countering habit-based behavior otherwise facilitated by developmental stress hormone exposure. They also reveal an early-life sensitive period during which trkB-ERK42/44 tone determines long-term behavioral outcomes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Patterson TK, Craske MG, Knowlton BJ. The effect of early-life stress on memory systems supporting instrumental behavior. Hippocampus. 2013;23(11):1025–34. doi: 10.1002/hipo.22174 - DOI - PubMed
-
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325(5940):621–5. doi: 10.1126/science.1171203 - DOI - PubMed
-
- Gourley SL, Swanson AM, Jacobs AM, Howell JL, Mo M, Dileone RJ, et al. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc Natl Acad Sci U S A. 2012;109(50):20714–9. doi: 10.1073/pnas.1208342109 - DOI - PMC - PubMed
-
- Guenzel FM, Wolf OT, Schwabe L. Glucocorticoids boost stimulus-response memory formation in humans. Psychoneuroendocrinology. 2014;45:21–30. doi: 10.1016/j.psyneuen.2014.02.015 - DOI - PubMed
-
- Schwabe L, Bohbot VD, Wolf OT. Prenatal stress changes learning strategies in adulthood. Hippocampus. 2012;22(11):2136–43. doi: 10.1002/hipo.22034 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

