Insulinlike Growth Factor-Binding Protein-1 Improves Vascular Endothelial Repair in Male Mice in the Setting of Insulin Resistance
- PMID: 29186427
- PMCID: PMC5776633
- DOI: 10.1210/en.2017-00572
Insulinlike Growth Factor-Binding Protein-1 Improves Vascular Endothelial Repair in Male Mice in the Setting of Insulin Resistance
Abstract
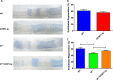
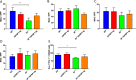
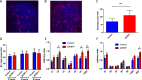
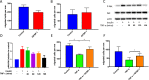
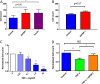
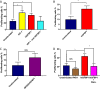
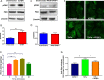
Insulin resistance is associated with impaired endothelial regeneration in response to mechanical injury. We recently demonstrated that insulinlike growth factor-binding protein-1 (IGFBP1) ameliorated insulin resistance and increased nitric oxide generation in the endothelium. In this study, we hypothesized that IGFBP1 would improve endothelial regeneration and restore endothelial reparative functions in the setting of insulin resistance. In male mice heterozygous for deletion of insulin receptors, endothelial regeneration after femoral artery wire injury was enhanced by transgenic expression of human IGFBP1 (hIGFBP1). This was not explained by altered abundance of circulating myeloid angiogenic cells. Incubation of human endothelial cells with hIGFBP1 increased integrin expression and enhanced their ability to adhere to and repopulate denuded human saphenous vein ex vivo. In vitro, induction of insulin resistance by tumor necrosis factor α (TNFα) significantly inhibited endothelial cell migration and proliferation. Coincubation with hIGFBP1 restored endothelial migratory and proliferative capacity. At the molecular level, hIGFBP1 induced phosphorylation of focal adhesion kinase, activated RhoA and modulated TNFα-induced actin fiber anisotropy. Collectively, the effects of hIGFBP1 on endothelial cell responses and acceleration of endothelial regeneration in mice indicate that manipulating IGFBP1 could be exploited as a putative strategy to improve endothelial repair in the setting of insulin resistance.
Copyright © 2018 Endocrine Society.
Figures

Similar articles
-
Increasing circulating IGFBP1 levels improves insulin sensitivity, promotes nitric oxide production, lowers blood pressure, and protects against atherosclerosis.Diabetes. 2012 Apr;61(4):915-24. doi: 10.2337/db11-0963. Epub 2012 Feb 22. Diabetes. 2012. PMID: 22357965 Free PMC article.
-
Haploinsufficiency of the insulin-like growth factor-1 receptor enhances endothelial repair and favorably modifies angiogenic progenitor cell phenotype.Arterioscler Thromb Vasc Biol. 2014 Sep;34(9):2051-8. doi: 10.1161/ATVBAHA.114.304121. Epub 2014 Jul 10. Arterioscler Thromb Vasc Biol. 2014. PMID: 25012136
-
Emodin Increases Expression of Insulin-Like Growth Factor Binding Protein 1 through Activation of MEK/ERK/AMPKα and Interaction of PPARγ and Sp1 in Lung Cancer.Cell Physiol Biochem. 2017;41(1):339-357. doi: 10.1159/000456281. Epub 2017 Jan 26. Cell Physiol Biochem. 2017. PMID: 28214826
-
Insulin resistance impairs circulating angiogenic progenitor cell function and delays endothelial regeneration.Diabetes. 2011 Apr;60(4):1295-303. doi: 10.2337/db10-1080. Epub 2011 Feb 11. Diabetes. 2011. PMID: 21317296 Free PMC article.
-
Effects of insulin resistance on endothelial progenitor cells and vascular repair.Clin Sci (Lond). 2009 Aug 3;117(5):173-90. doi: 10.1042/CS20080263. Clin Sci (Lond). 2009. PMID: 19630751 Review.
Cited by
-
Plasma human growth cytokines in children with vasovagal syncope.Front Cardiovasc Med. 2022 Oct 14;9:1030618. doi: 10.3389/fcvm.2022.1030618. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36312268 Free PMC article.
-
Plasma proteomics and lipidomics facilitate elucidation of the link between Alzheimer's disease development and vessel wall fragility.Sci Rep. 2024 Aug 27;14(1):19901. doi: 10.1038/s41598-024-71097-9. Sci Rep. 2024. PMID: 39191863 Free PMC article.
-
Preservation of vascular endothelial repair in mice with diet-induced obesity.Obes Sci Pract. 2018 Jun 26;4(5):490-496. doi: 10.1002/osp4.282. eCollection 2018 Oct. Obes Sci Pract. 2018. PMID: 30338120 Free PMC article.
-
Signaling Pathways of the Insulin-like Growth Factor Binding Proteins.Endocr Rev. 2023 Sep 15;44(5):753-778. doi: 10.1210/endrev/bnad008. Endocr Rev. 2023. PMID: 36974712 Free PMC article.
-
Research Advances in the Roles of Circular RNAs in Pathophysiology and Early Diagnosis of Gestational Diabetes Mellitus.Front Cell Dev Biol. 2022 Jan 4;9:739511. doi: 10.3389/fcell.2021.739511. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35059395 Free PMC article. Review.
References
-
- Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med (Berl). 2004;82(10):671–677. - PubMed
-
- Manchio JV, Gu J, Romar L, Brown J, Gammie J, Pierson RN III, Griffith B, Poston RS. Disruption of graft endothelium correlates with early failure after off-pump coronary artery bypass surgery. Ann Thorac Surg. 2005;79(6):1991–1998. - PubMed
-
- Kipshidze N, Dangas G, Tsapenko M, Moses J, Leon MB, Kutryk M, Serruys P. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J Am Coll Cardiol. 2004;44(4):733–739. - PubMed
-
- Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115(18):2435–2441. - PubMed
-
- Haudenschild CC, Schwartz SM. Endothelial regeneration. II. Restitution of endothelial continuity. Lab Invest. 1979;41(5):407–418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

