Autophagy in Measles Virus Infection
- PMID: 29186766
- PMCID: PMC5744134
- DOI: 10.3390/v9120359
Autophagy in Measles Virus Infection
Abstract
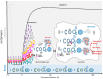
Autophagy is a biological process that helps cells to recycle obsolete cellular components and which greatly contributes to maintaining cellular integrity in response to environmental stress factors. Autophagy is also among the first lines of cellular defense against invading microorganisms, including viruses. The autophagic destruction of invading pathogens, a process referred to as xenophagy, involves cytosolic autophagy receptors, such as p62/SQSTM1 (Sequestosome 1) or NDP52/CALCOCO2 (Nuclear Dot 52 KDa Protein/Calcium Binding And Coiled-Coil Domain 2), which bind to microbial components and target them towards growing autophagosomes for degradation. However, most, if not all, infectious viruses have evolved molecular tricks to escape from xenophagy. Many viruses even use autophagy, part of the autophagy pathway or some autophagy-associated proteins, to improve their infectious potential. In this regard, the measles virus, responsible for epidemic measles, has a unique interface with autophagy as the virus can induce multiple rounds of autophagy in the course of infection. These successive waves of autophagy result from distinct molecular pathways and seem associated with anti- and/or pro-measles virus consequences. In this review, we describe what the autophagy-measles virus interplay has taught us about both the biology of the virus and the mechanistic orchestration of autophagy.
Keywords: CD46; IRGM; NDP52; OPTN; T6BP; autophagy; autophagy receptors; measles virus; selective autophagy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Tatsuo H., Ono N., Tanaka K., Yanagi Y. Slam (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

