Species-Specific Inactivation of Triosephosphate Isomerase from Trypanosoma brucei: Kinetic and Molecular Dynamics Studies
- PMID: 29186784
- PMCID: PMC6149853
- DOI: 10.3390/molecules22122055
Species-Specific Inactivation of Triosephosphate Isomerase from Trypanosoma brucei: Kinetic and Molecular Dynamics Studies
Abstract
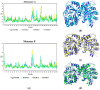
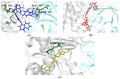
Human African Trypanosomiasis (HAT), a disease that provokes 2184 new cases a year in Sub-Saharan Africa, is caused by Trypanosoma brucei. Current treatments are limited, highly toxic, and parasite strains resistant to them are emerging. Therefore, there is an urgency to find new drugs against HAT. In this context, T. brucei depends on glycolysis as the unique source for ATP supply; therefore, the enzyme triosephosphate isomerase (TIM) is an attractive target for drug design. In the present work, three new benzimidazole derivatives were found as TbTIM inactivators (compounds 1, 2 and 3) with an I50 value of 84, 82 and 73 µM, respectively. Kinetic analyses indicated that the three molecules were selective when tested against human TIM (HsTIM) activity. Additionally, to study their binding mode in TbTIM, we performed a 100 ns molecular dynamics simulation of TbTIM-inactivator complexes. Simulations showed that the binding of compounds disturbs the structure of the protein, affecting the conformations of important domains such as loop 6 and loop 8. In addition, the physicochemical and drug-like parameters showed by the three compounds suggest a good oral absorption. In conclusion, these molecules will serve as a guide to design more potent inactivators that could be used to obtain new drugs against HAT.
Keywords: enzymatic kinetics; human African trypanosomiasis; molecular dynamics simulation; triosephosphate isomerase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- W.H.O. Trypanosomiasis, Human African (Sleeping Sickness), Epidemiological Situation. [(accessed on 17 September 2017)]; Available online: http://www.who.int/trypanosomiasis_african/country/en/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

