Impact of Antibiotics on the Proliferation and Differentiation of Human Adipose-Derived Mesenchymal Stem Cells
- PMID: 29186789
- PMCID: PMC5751125
- DOI: 10.3390/ijms18122522
Impact of Antibiotics on the Proliferation and Differentiation of Human Adipose-Derived Mesenchymal Stem Cells
Abstract
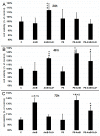
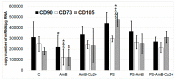
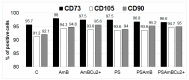
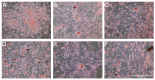
Adipose tissue is a promising source of mesenchymal stem cells. Their potential to differentiate and regenerate other types of tissues may be affected by several factors. This may be due to in vitro cell-culture conditions, especially the supplementation with antibiotics. The aim of our study was to evaluate the effects of a penicillin-streptomycin mixture (PS), amphotericin B (AmB), a complex of AmB with copper (II) ions (AmB-Cu2+) and various combinations of these antibiotics on the proliferation and differentiation of adipose-derived stem cells in vitro. Normal human adipose-derived stem cells (ADSC, Lonza) were routinely maintained in a Dulbecco's Modified Eagle Medium (DMEM) that was either supplemented with selected antibiotics or without antibiotics. The ADSC that were used for the experiment were at the second passage. The effect of antibiotics on proliferation was analyzed using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) and sulforhodamine-B (SRB) tests. Differentiation was evaluated based on Alizarin Red staining, Oil Red O staining and determination of the expression of ADSC, osteoblast and adipocyte markers by real-time RT-qPCR. The obtained results indicate that the influence of antibiotics on adipose-derived stem cells depends on the duration of exposure and on the combination of applied compounds. We show that antibiotics alter the proliferation of cells and also promote natural osteogenesis, and adipogenesis, and that this effect is also noticeable in stimulated osteogenesis.
Keywords: adipose-derived stem cells; amphotericin B; copper (II) ions; osteogenesis; penicillin; streptomycin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Cieslar-Pobuda A., Knoflach V., Ringh M.V., Stark J., Likus W., Siemianowicz K., Ghavami S., Hudecki A., Green J.L., Los M.J. Transdifferentiation and reprogramming: Overview of the processes, their similarities and differences. Biochim. Biophys. Acta. 2017;1864:1359–1369. doi: 10.1016/j.bbamcr.2017.04.017. - DOI - PubMed
-
- Baglioni S., Francalanci M., Squecco R., Lombardi A., Cantini G., Angeli R., Gelmini S., Guasti D., Benvenuti S., Annunziato F., et al. Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J. 2009;23:3494–3505. doi: 10.1096/fj.08-126946. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

