Upconversion Luminescence Sensitized pH-Nanoprobes
- PMID: 29186844
- PMCID: PMC6149687
- DOI: 10.3390/molecules22122064
Upconversion Luminescence Sensitized pH-Nanoprobes
Abstract
Photon upconversion materials, featuring excellent photophysical properties, are promising for bio-medical research due to their low autofluorescence, non-cytotoxicity, low photobleaching and high photostability. Upconversion based pH-nanoprobes are attracting considerable interest due to their superiority over pH-sensitive molecular indicators and metal nanoparticles. Herein, we review the advances in upconversion based pH-nanoprobes, the first time in the seven years since their discovery in 2009. With a brief discussion on the upconversion materials and upconversion processes, the progress in this field has been overviewed, along with the toxicity and biodistribution of upconversion materials for intracellular application. We strongly believe that this survey will encourage the further pursuit of intense research for designing molecular pH-sensors.
Keywords: molecular probes; optical sensors; pH-sensors; photoluminescence; upconversion.
Conflict of interest statement
There is no conflict of interests.
Figures

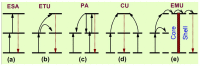

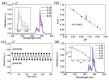
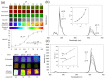
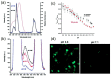
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

