Design, Synthesis and Bioactivities of Novel Isoxazole-Containing Pyrazole Oxime Derivatives
- PMID: 29186906
- PMCID: PMC6149770
- DOI: 10.3390/molecules22122000
Design, Synthesis and Bioactivities of Novel Isoxazole-Containing Pyrazole Oxime Derivatives
Abstract
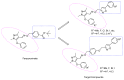
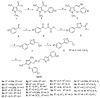
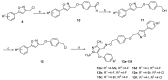
In this study, in order to find novel biologically active pyrazole oxime derivatives, twenty-eight new pyrazole oxime compounds containing a substituted isoxazole ring were synthesized and evaluated for their acaricidaland insecticidal activities. Bioassays exhibited that some target compounds indicated good acaricidal and insecticidal activities against Tetranychus cinnabarinus, Aphis medicaginis, Mythimna separata, and Nilaparvata lugens. Especially, compounds 9c, 9h, 9u, and 9v showed 100.00%, 90.56%, 90.78%, and 90.62% insecticidal activities against A. medicaginis at the concentration of 20 μg/mL, respectively, compounds 9k and 9u had 70.86% and 100.00% insecticidal activities against M. separata at 20 μg/mL, respectively.
Keywords: bioactivity; isoxazole; pyrazole oxime; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

