A mobile and web-based clinical decision support and monitoring system for diabetes mellitus patients in primary care: a study protocol for a randomized controlled trial
- PMID: 29187186
- PMCID: PMC5707797
- DOI: 10.1186/s12911-017-0558-6
A mobile and web-based clinical decision support and monitoring system for diabetes mellitus patients in primary care: a study protocol for a randomized controlled trial
Abstract
Background: Physicians' guideline use rates for diagnosis, treatment and monitoring of diabetes mellitus (DM) is very low. Time constraints, patient overpopulation, and complex guidelines require alternative solutions for real time patient monitoring. Rapidly evolving e-health technology combined with clinical decision support and monitoring systems (CDSMS) provides an effective solution to these problems. The purpose of the study is to develop a user-friendly, comprehensive, fully integrated web and mobile-based Clinical Decision Support and Monitoring System (CDSMS) for the screening, diagnosis, treatment, and monitoring of DM diseases which is used by physicians and patients in primary care and to determine the effectiveness of the system.
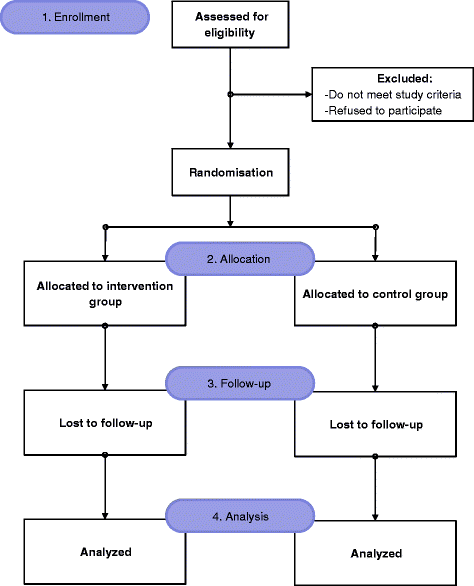
Methods: The CDSMS will be based on evidence-based guidelines for DM disease. A web and mobile-based application will be developed in which the physician will remotely monitor patient data through mobile applications in real time. The developed CDSMS will be tested in two stages. In the first stage, the usability, understandability, and adequacy of the application will be determined. Five primary care physicians will use the developed application for at least 16 DM patients. Necessary improvements will be made according to physician feedback. In the second phase, a parallel, single-blind, randomized controlled trial will be implemented. DM diagnosed patients will be recruited for the CDSMS trial by their primary care physicians. Ten physicians and their 439 patients will be involved in the study. Eligible participants will be assigned to intervention and control groups with simple randomization. The significance level will be accepted as p < 0.05. In the intervention group, the system will make recommendations on patient monitoring, diagnosis, and treatment. These recommendations will be implemented at the physician's discretion. Patients in the control group will be treated by physicians according to current DM treatment standards. Patients in both groups will be monitored for 6 months. Patient data will be compared between 0th and 6th month of the study. . Clinical and laboratory outcomes will be assessed in person while others will be self-assessed online.
Discussion: The developed system will be the first of its kind to utilize evidence based guidelines to provide health services to DM patients.
Trial registration: ClinicalTrials.gov NCT02917226 . 28 September 2016.
Keywords: Clinical decision support; Diabetes; E-health; M-health.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval to conduct this project was granted by the Dokuz Eylül University Ethics Committee with reference number 2015/23–40. Written informed consent will be obtained from the participants of the trial. The informed consent form was approved by Dokuz Eylül University Ethics Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Sağlık Bakanlığı TC. Ulusal Hastalık Yükü Çalışması. 2004.
-
- Ilkova H, Damci T, Siva ZO, et al. Antidiyabetik İlaç Kullanım Paternlerindeki Değişim ile Tip 2 Diabetes Mellituslu Hastalarda HbA1c Düzeyi Arasındaki İlişki: Türkiye’de Son 20 Yılda Yapılan Çalışmaların Sistematik Analizi. Turkish J Endocrinol Metab. 2011;15(4):77–105.
-
- Aggeleton P, Chalmers H. Models and theories five: Orem’s self-care model. Nurs Times. 1985;2:36–39. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical