Atypical, non-standard functions of the microtubule associated Tau protein
- PMID: 29187252
- PMCID: PMC5707803
- DOI: 10.1186/s40478-017-0489-6
Atypical, non-standard functions of the microtubule associated Tau protein
Abstract
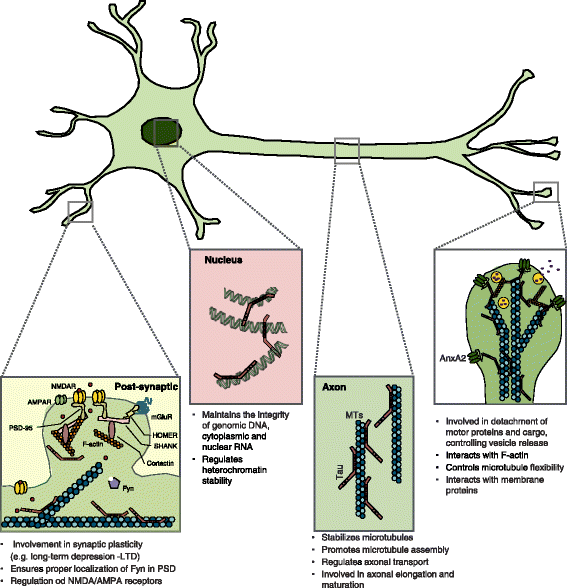
Since the discovery of the microtubule-associated protein Tau (MAPT) over 40 years ago, most studies have focused on Tau's role in microtubule stability and regulation, as well as on the neuropathological consequences of Tau hyperphosphorylation and aggregation in Alzheimer's disease (AD) brains. In recent years, however, research efforts identified new interaction partners and different sub-cellular localizations for Tau suggesting additional roles beyond its standard function as microtubule regulating protein. Moreover, despite the increasing research focus on AD over the last decades, Tau was only recently considered as a promising therapeutic target for the treatment and prevention of AD as well as for neurological pathologies beyond AD e.g. epilepsy, excitotoxicity, and environmental stress. This review will focus on atypical, non-standard roles of Tau on neuronal function and dysfunction in AD and other neurological pathologies providing novel insights about neuroplastic and neuropathological implications of Tau in both the central and the peripheral nervous system.
Keywords: Alzheimer’s disease; Dendrites; Neuronal function; Nucleus; Pathology; Subcellular localization; Synapse; Tau; Tau isoform.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical