Initial nutritional management during noninvasive ventilation and outcomes: a retrospective cohort study
- PMID: 29187261
- PMCID: PMC5707783
- DOI: 10.1186/s13054-017-1867-y
Initial nutritional management during noninvasive ventilation and outcomes: a retrospective cohort study
Abstract
Background: Patients starting noninvasive ventilation (NIV) to treat acute respiratory failure are often unable to eat and therefore remain in the fasting state or receive nutritional support. Maintaining a good nutritional status has been reported to improve patient outcomes. In the present study, our primary objective was to describe the nutritional management of patients starting first-line NIV, and our secondary objectives were to assess potential associations between nutritional management and outcomes.
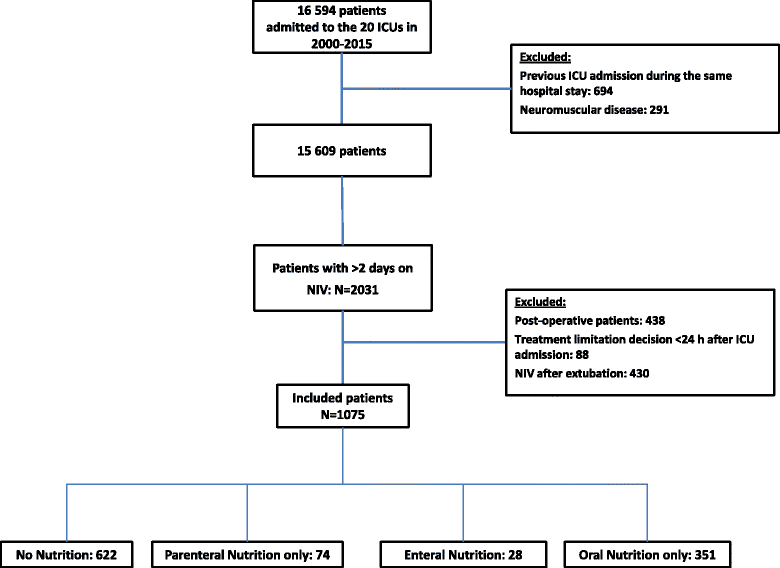
Methods: Observational retrospective cohort study of a prospective database fed by 20 French intensive care units. Adult medical patients receiving NIV for more than 2 consecutive days were included and divided into four groups on the basis of nutritional support received during the first 2 days of NIV: no nutrition, enteral nutrition, parenteral nutrition only, and oral nutrition only.
Results: Of the 16,594 patients admitted during the study period, 1075 met the inclusion criteria; of these, 622 (57.9%) received no nutrition, 28 (2.6%) received enteral nutrition, 74 (6.9%) received parenteral nutrition only, and 351 (32.7%) received oral nutrition only. After adjustment for confounders, enteral nutrition (vs. no nutrition) was associated with higher 28-day mortality (adjusted HR, 2.3; 95% CI, 1.2-4.4) and invasive mechanical ventilation needs (adjusted HR, 2.1; 95% CI, 1.1-4.2), as well as with fewer ventilator-free days by day 28 (adjusted relative risk, 0.7; 95% CI, 0.5-0.9).
Conclusions: Nearly three-fifths of patients receiving NIV fasted for the first 2 days. Lack of feeding or underfeeding was not associated with mortality. The optimal route of nutrition for these patients needs to be investigated.
Keywords: Acute respiratory failure; Intensive care unit; Noninvasive mechanical ventilation; Nutrition; Pneumonia.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Clermont-Ferrand Hospital institutional review board (Clinical Investigation Center Ethics Committee [CECIC] Clermont-Ferrand IRB number 5891, reference 2007-16), which waived the need for written informed consent of the participants, in accordance with French legislation on noninterventional studies.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Demoule A, Chevret S, Carlucci A, Kouatchet A, Jaber S, Meziani F, Schmidt M, Schnell D, Clergue C, Aboab J, et al. Changing use of noninvasive ventilation in critically ill patients: trends over 15 years in francophone countries. Intensive Care Med. 2016;42(1):82–92. doi: 10.1007/s00134-015-4087-4. - DOI - PubMed
-
- Schnell D, Timsit JF, Darmon M, Vesin A, Goldgran-Toledano D, Dumenil AS, Garrouste-Orgeas M, Adrie C, Bouadma L, Planquette B, et al. Noninvasive mechanical ventilation in acute respiratory failure: trends in use and outcomes. Intensive Care Med. 2014;40(4):582–91. doi: 10.1007/s00134-014-3222-y. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous