Vitamin D supplementation during pregnancy: state of the evidence from a systematic review of randomised trials
- PMID: 29187358
- PMCID: PMC5706533
- DOI: 10.1136/bmj.j5237
Vitamin D supplementation during pregnancy: state of the evidence from a systematic review of randomised trials
Abstract
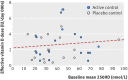
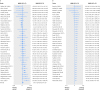
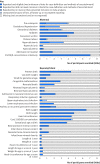
Objectives To estimate the effects of vitamin D supplementation during pregnancy on 11 maternal and 27 neonatal/infant outcomes; to determine frequencies at which trial outcome data were missing, unreported, or inconsistently reported; and to project the potential contributions of registered ongoing or planned trials.Design Systematic review and meta-analysis of randomised controlled trials; systematic review of registered but unpublished trials.Data sources Medline, Embase, PubMed, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials from inception to September 2017; manual searches of reference lists of systematic reviews identified in the electronic search; and online trial registries for unpublished, ongoing, or planned trials.Eligibility criteria for study selection Trials of prenatal vitamin D supplementation with randomised allocation and control groups administered placebo, no vitamin D, or vitamin D ≤600 IU/day (or its equivalent), and published in a peer reviewed journal.Results 43 trials (8406 participants) were eligible for meta-analyses. Median sample size was 133 participants. Vitamin D increased maternal/cord serum concentration of 25-hydroxyvitamin D, but the dose-response effect was weak. Maternal clinical outcomes were rarely ascertained or reported, but available data did not provide evidence of benefits. Overall, vitamin D increased mean birth weight of 58.33 g (95% confidence interval 18.88 g to 97.78 g; 37 comparisons) and reduced the risk of small for gestational age births (risk ratio 0.60, 95% confidence interval 0.40 to 0.90; seven comparisons), but findings were not robust in sensitivity and subgroup analyses. There was no effect on preterm birth (1.0, 0.77 to 1.30; 15 comparisons). There was strong evidence that prenatal vitamin D reduced the risk of offspring wheeze by age 3 years (0.81, 0.67 to 0.98; two comparisons). For most outcomes, meta-analyses included data from a minority of trials. Only eight of 43 trials (19%) had an overall low risk of bias. Thirty five planned/ongoing randomised controlled trials could contribute 12 530 additional participants to future reviews.Conclusions Most trials on prenatal vitamin D published by September 2017 were small and of low quality. The evidence to date seems insufficient to guide clinical or policy recommendations. Future trials should be designed and powered to examine clinical endpoints, including maternal conditions related to pregnancy (such as pre-eclampsia), infant growth, and respiratory outcomes.Systematic review registration PROSPERO CRD42016051292.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
-
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D.The National Academies Press, 2010. - PubMed
-
- Zhang R, Li B, Gao X, et al. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am J Clin Nutr 2017;105:810-9. 10.3945/ajcn.116.140392 pmid:28251933. - DOI - PubMed
-
- Keum N, Giovannucci E. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. Br J Cancer 2014;111:976-80. 10.1038/bjc.2014.294 pmid:24918818. - DOI - PMC - PubMed
-
- Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356:i6583 10.1136/bmj.i6583 pmid:28202713. - DOI - PMC - PubMed
-
- Martineau AR, Cates CJ, Urashima M, et al. Vitamin D for the management of asthma. Cochrane Database Syst Rev 2016;9:CD011511.pmid:27595415. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
