Protocol for the melatools skin self-monitoring trial: a phase II randomised controlled trial of an intervention for primary care patients at higher risk of melanoma
- PMID: 29187412
- PMCID: PMC5719271
- DOI: 10.1136/bmjopen-2017-017934
Protocol for the melatools skin self-monitoring trial: a phase II randomised controlled trial of an intervention for primary care patients at higher risk of melanoma
Abstract
Introduction: Melanoma is the fifth most common cancer in the UK. Incidence rates have quadrupled over the last 30 years and continue to rise, especially among younger people. As routine screening of the general population is not currently recommended in the UK, a focus on secondary prevention through early detection and prompt treatment in individuals at increased risk of melanoma could make an important contribution to improve melanoma outcomes. This paper describes the protocol for a phase II, multisite, randomised controlled trial, in the primary care setting, for patients at increased risk of melanoma. A skin self-monitoring (SSM) smartphone 'App' was used to improve symptom appraisal and encourage help seeking in primary care, thereby promoting early presentation with skin changes suspicious of melanoma.
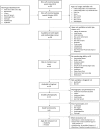
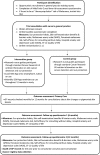
Methods and analysis: We aim to recruit 200 participants from general practice waiting rooms in the East of England. Eligible patients are those identified at higher melanoma risk (using a real-time risk assessment tool), without a personal history of melanoma, aged 18 to 75 years. Participants will be invited to a primary care nurse consultation, and randomised to the intervention group (standard written advice on skin cancer detection and sun protection, loading of an SSM 'App' onto the participant's smartphone and instructions on use including self-monitoring reminders) or control group (standard written advice alone). The primary outcomes are consultation rates for changes to a pigmented skin lesion, and the patient interval (time from first noticing a skin change to consultation). Secondary outcomes include patient sun protection behaviours, psychosocial outcomes, and measures of trial feasibility and acceptability.
Ethics and dissemination: NHS ethical approval has been obtained from Cambridgeshire and Hertfordshire research ethics committee (REC reference 16/EE/0248). The findings from the MelaTools SSM Trial will be disseminated widely through peer-reviewed publications and scientific conferences.
Trial registration number: ISCTRN16061621.
Keywords: melanoma; primary care; randomised controlled trial; risk-stratified; skin cancer.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Effect of a Skin Self-monitoring Smartphone Application on Time to Physician Consultation Among Patients With Possible Melanoma: A Phase 2 Randomized Clinical Trial.JAMA Netw Open. 2020 Feb 5;3(2):e200001. doi: 10.1001/jamanetworkopen.2020.0001. JAMA Netw Open. 2020. PMID: 32101302 Free PMC article. Clinical Trial.
-
Protocol for the CHEST Australia Trial: a phase II randomised controlled trial of an intervention to reduce time-to-consult with symptoms of lung cancer.BMJ Open. 2015 May 18;5(5):e008046. doi: 10.1136/bmjopen-2015-008046. BMJ Open. 2015. PMID: 25986641 Free PMC article. Clinical Trial.
-
A prospective, randomized, single-blinded, crossover trial to investigate the effect of a wearable device in addition to a daily symptom diary for the remote early detection of SARS-CoV-2 infections (COVID-RED): a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 Jun 22;22(1):412. doi: 10.1186/s13063-021-05241-5. Trials. 2021. PMID: 34158099 Free PMC article.
-
Behavioral Counseling for Skin Cancer Prevention: A Systematic Evidence Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Mar. Report No.: 17-05234-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Mar. Report No.: 17-05234-EF-1. PMID: 29697227 Free Books & Documents. Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
Hypoxemia during procedural sedation in adult patients: a retrospective observational study.Can J Anaesth. 2021 Sep;68(9):1349-1357. doi: 10.1007/s12630-021-01992-6. Epub 2021 Apr 20. Can J Anaesth. 2021. PMID: 33880728 Free PMC article.
-
Melanoma Epidemiology and Early Detection in Europe: Diversity and Disparities.Dermatol Pract Concept. 2020 Jun 29;10(3):e2020033. doi: 10.5826/dpc.1003a33. eCollection 2020 Jul. Dermatol Pract Concept. 2020. PMID: 32642304 Free PMC article. Review.
-
Prevalence and Age-Related Patterns in Health Information-Seeking Behaviors and Technology Use Among Skin Cancer Survivors: Survey Study.JMIR Dermatol. 2022 Apr-Jun;5(2):e36256. doi: 10.2196/36256. Epub 2022 Apr 22. JMIR Dermatol. 2022. PMID: 36776536 Free PMC article.
-
Effect of a Skin Self-monitoring Smartphone Application on Time to Physician Consultation Among Patients With Possible Melanoma: A Phase 2 Randomized Clinical Trial.JAMA Netw Open. 2020 Feb 5;3(2):e200001. doi: 10.1001/jamanetworkopen.2020.0001. JAMA Netw Open. 2020. PMID: 32101302 Free PMC article. Clinical Trial.
-
Is high-flow safer than low-flow nasal oxygenation for procedural sedation?Can J Anaesth. 2021 Apr;68(4):439-444. doi: 10.1007/s12630-020-01884-1. Epub 2021 Jan 12. Can J Anaesth. 2021. PMID: 33432498 English. No abstract available.
References
-
- Cancer Research UK website.http://www.cancerresearchuk.org/health-professional/cancer-statistics/st... (accessed 6 May 2017).
-
- Cancer Research UK website. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/skin/survi... (accessed 6 May 2017).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials